Abstract
The requirement of initiation factors F1 (highly purified) and F2 (electrophoretically homogeneous) for ribosomal binding of N-formylmethionyl transfer RNA (fMet ∼ tRNA) at low Mg2+ concentration (3.5 mM), with the trinucleoside diphosphate ApUpG as messenger, was studied under various experimental conditions with 30S + 50S ribosomes and with 30S subunits alone. The results were qualitatively the same in both cases but the amount of binding was two to three times higher when both 30S and 50S subunits were present.
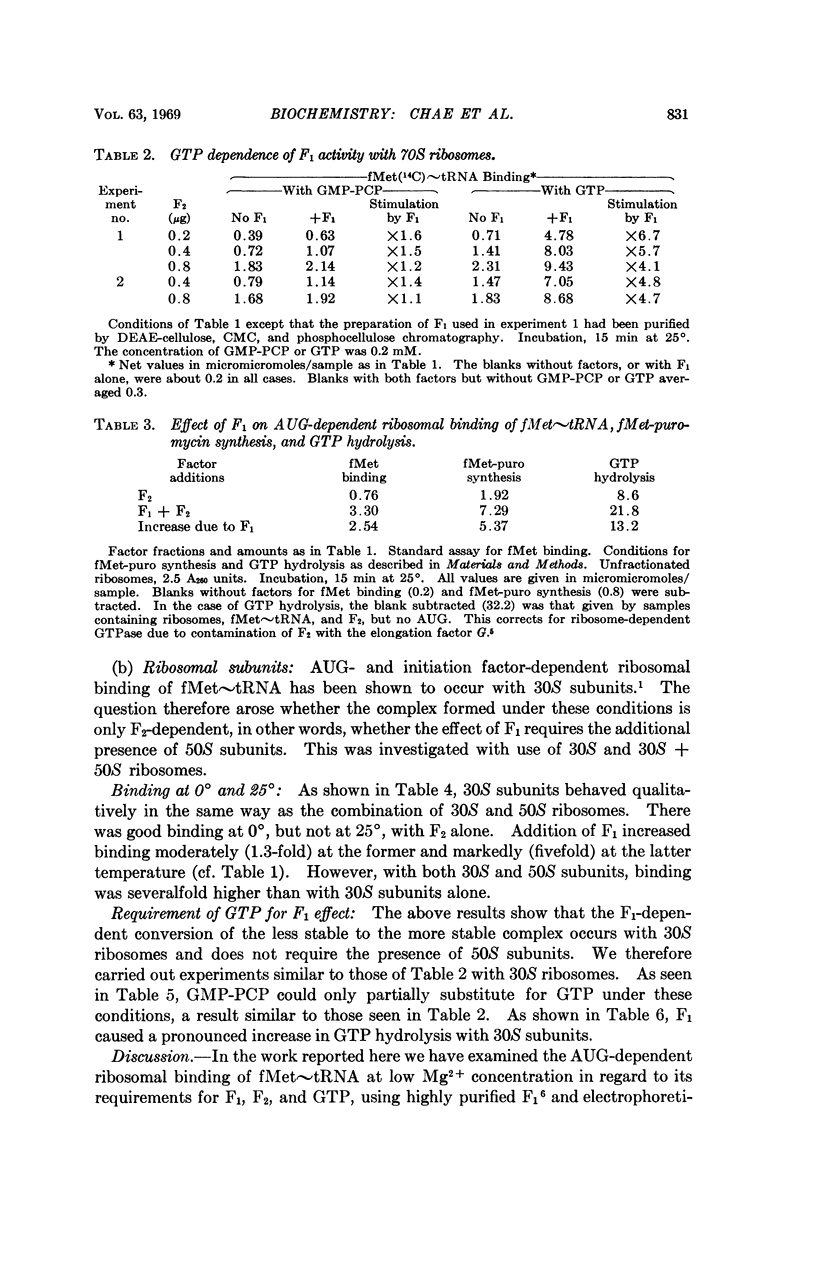
Although there was a virtually absolute requirement for F2 in all cases, considerable binding occurred at 0° in the absence of added F1. F1 addition stimulated binding up to twofold under these conditions. However, at 25°, the temperature at which the reaction is usually carried out, there was very little binding with F2 alone and addition of F1 stimulated the reaction five- to sixfold.
Contrary to current belief, the GTP analog 5′-guanylyldiphosphonate (GMP-PCP) cannot replace GTP in the binding reaction. In particular, there was but little stimulation of binding (about 1.5-fold) by addition of F1 to F2-containing samples when GMP-PCP was used. In marked contrast, binding was stimulated up to sevenfold by addition of F1 when GTP was substituted for the analog. Under these conditions, there was an ApUpG and F1-dependent hydrolysis of GTP. This is observable with 30S subunits alone and can hardly be related to the occurrence of translocation.
The results may be interpreted to mean that a complex relatively stable at 0°, but less stable at 25°, is formed upon addition of F2 alone. Conversion of the less stable to the more stable form of complex is made possible by addition of F1. This is accompanied or mediated by cleavage of GTP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chae Y. B., Mazumder R., Ochoa S. Polypeptide chain initiation in E. coli: isolation of homogeneous initiation factor E2 and its relation to ribosomal proteins. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1181–1188. doi: 10.1073/pnas.62.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille M. B., Miller M. J., Iwasaki K., Wahba A. J. Translation of the genetic message. VI. The role of ribosomal subunits in binding of formylmethionyl-tRNA and its reaction with puromycin. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1652–1654. doi: 10.1073/pnas.58.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Dewey K. F., Hershey J. W., Thach R. E. Guanosine 5'-triphosphatase activity of initiation factor f2. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1066–1070. doi: 10.1073/pnas.61.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis II. A convenient assay for the ribosome-dependent synthesis of N-formyl-C14-methionylpuromycin. Biochem Biophys Res Commun. 1966 Oct 20;25(2):233–238. doi: 10.1016/0006-291x(66)90586-9. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Zasloff M., Ochoa S. Association of polypeptide initiation factors with 30 S ribosomal subunits. FEBS Lett. 1969 Apr;3(1):50–52. doi: 10.1016/0014-5793(69)80094-3. [DOI] [PubMed] [Google Scholar]
- Mukundan M. A., Hershey J. W., Dewey K. F., Thach R. E. Binding of formylmethionyl-tRNA to 30S ribosomal sub-units. Nature. 1968 Mar 16;217(5133):1013–1016. doi: 10.1038/2171013a0. [DOI] [PubMed] [Google Scholar]
- Ochoa S. Translation of the genetic message. Naturwissenschaften. 1968 Nov;55(11):505–514. doi: 10.1007/BF00660121. [DOI] [PubMed] [Google Scholar]
- Parenti-Rosina R., Eisenstadt A., Eisenstadt J. M. Isolation of protein initiation factors from 30S ribosomal subunits. Nature. 1969 Jan 25;221(5178):363–365. doi: 10.1038/221363a0. [DOI] [PubMed] [Google Scholar]
- Revel M., Lelong J. C., Brawerman G., Gros F. Function of three protein factors and ribosomal subunits in the initiation of protein synthesis in E. coli. Nature. 1968 Sep 7;219(5158):1016–1021. doi: 10.1038/2191016a0. [DOI] [PubMed] [Google Scholar]
- Salas M., Miller M. J., Wahba A. J., Ochoa S. Translation of the genetic message. V. Effect of Mg++ and formylation of methionine in protein synthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1865–1869. doi: 10.1073/pnas.57.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z., Zamir A., Elson D. On the specificity and stability of an enzyme that hydrolyzes N-substituted aminoacyl-transfer RNA's. Proc Natl Acad Sci U S A. 1968 Oct;61(2):701–707. doi: 10.1073/pnas.61.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]


