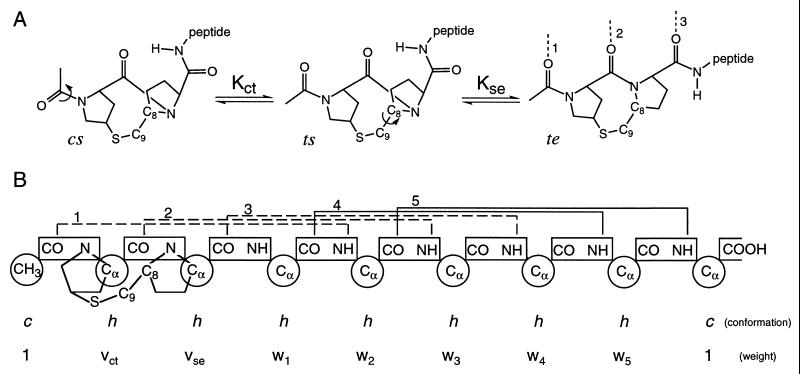
Figure 1.
(A) Schematic representation of the conformational transitions of the template required for helix nucleation. Cis–trans isomerization of the first peptide bond orients two carbonyl groups relative to one another, whereas rotation about the C8—C9 bond aligns the third carbonyl. In the te conformation, three carbonyl groups are aligned to accept helical hydrogen bonds, shown by dotted lines and numbered according to the propagating helical residue with which they are associated. (B) Diagram of unit conformations and weights assigned for the completely helical (te5) state of Ac-Hel1–A6-OH. Chain units are indicated by circles, and peptide bonds are shown in rectangles. The h and c conformations assigned to amino acid units indicate the helical and nonhelical conformations, respectively. Conformations assigned to template units represent the conformational state of the template (see Methods). Statistical weights are assigned based on unit conformations (see Methods and the Supplementary Material). i, i + 4 helical hydrogen bonds are indicated by brackets above the peptide bonds and numbered according to the propagating helical residue with which they are associated. Peptide–template hydrogen bonds are shown as dotted lines. Solid lines indicate intrapeptide hydrogen bonds.