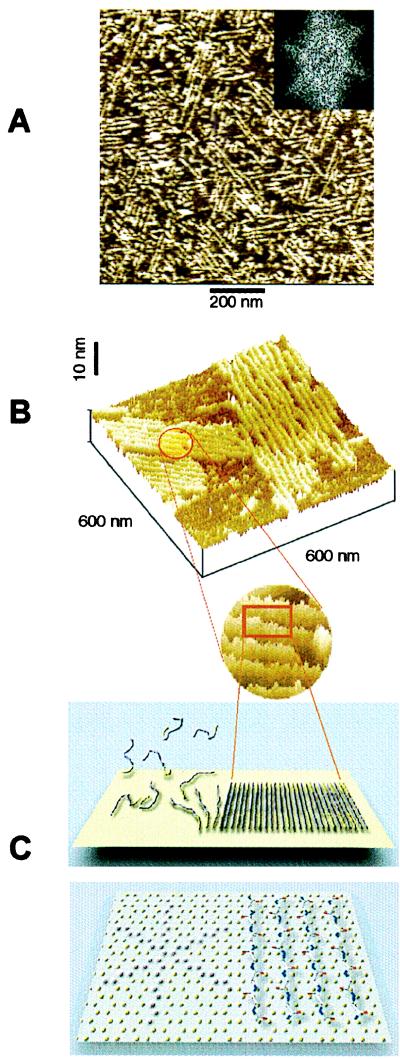
Figure 6.
(A) An example of preferential orientation of sheet-like aggregates of Aβ and their assemblies forming on graphite. The orientation of aggregates along the three directions at 120° to one another is reflected in the characteristic 6-fold symmetry of the two-dimensional Fourier transform of the image (Inset). The average lateral spacing between the sheets, determined from the radial position of spots in Fourier transform patterns of images from different experiments, was equal to 18.8 ± 1.8 nm. (B) Higher magnification view of two assemblies of Aβ aggregates on graphite (top) and a schematic model illustrating the orientation of peptide chains in the aggregates based on their dimensions (bottom). The height of the aggregates above the graphite surface, measured from their profiles ranged from 1.0 to 1.2 nm. Dimensions of Aβ aggregates on graphite provide a strong indication that they have a β-sheet character, with peptide chains perpendicular to the aggregate long axis. (C) The properly scaled model of peptide backbones in an antiparallel β-sheet arrangement, superimposed on the crystal structure of graphite surface. We propose that the orientation of Aβ aggregates on graphite is driven by a hydrophobic effect and reflects an attempt of the peptide chains to cover the rows of the most densely packed carbon atoms (highlighted in the left portion of the drawing).