Abstract
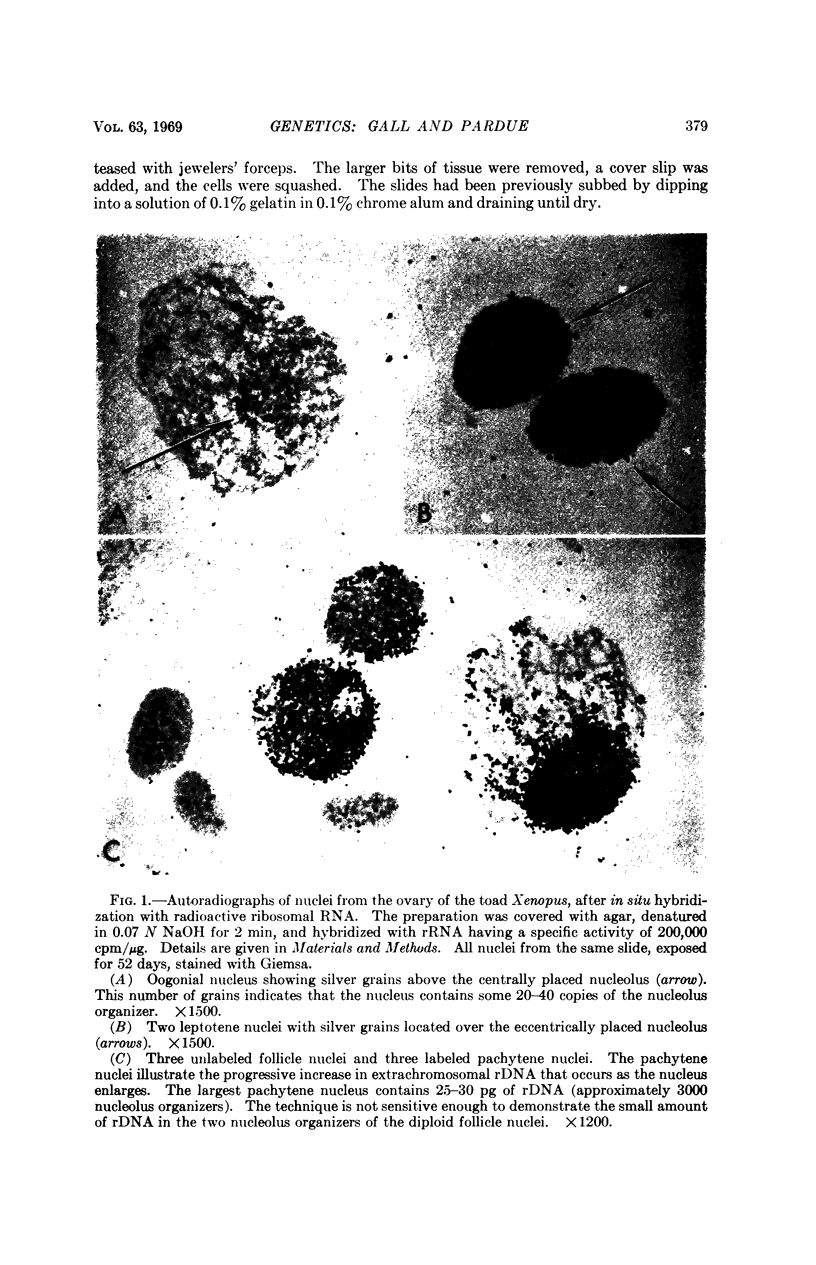
A technique is described for forming molecular hybrids between RNA in solution and the DNA of intact cytological preparations. Cells in a conventional tissue squash are immobilized under a thin layer of agar. Next they are treated with alkali to denature the DNA and then incubated with tritium-labeled RNA. The hybrids are detected by autoradiography. The technique is illustrated by the hybridization of ribosomal RNA to the amplified ribosomal genes in oocytes of the toad Xenopus. A low level of gene amplification was also detected in premeiotic nuclei (oogonia).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUTZ E. K., HALL B. D. The isolation of T4-specific RNA on a DNA-cellulose column. Proc Natl Acad Sci U S A. 1962 Mar 15;48:400–408. doi: 10.1073/pnas.48.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON E. T., McCARTHY B. J. A general method for the isolation of RNA complementary to DNA. Proc Natl Acad Sci U S A. 1962 Aug;48:1390–1397. doi: 10.1073/pnas.48.8.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- CONGER A. D., FAIRCHILD L. M. A quick-freeze method for making smear slides permanent. Stain Technol. 1953 Nov;28(6):281–283. doi: 10.3109/10520295309105555. [DOI] [PubMed] [Google Scholar]
- Evans D., Birnstiel M. L. Localization of amplified ribosomal DNA in the oocyte of Xenopus laevis. Biochim Biophys Acta. 1968 Aug 23;166(1):274–276. doi: 10.1016/0005-2787(68)90517-0. [DOI] [PubMed] [Google Scholar]
- Flamm W. G., McCallum M., Walker P. M. The isolation of complementary strands from a mouse DNA fraction. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1729–1734. doi: 10.1073/pnas.57.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- HALL B. D., SPIEGELMAN S. Sequence complementarity of T2-DNA and T2-specific RNA. Proc Natl Acad Sci U S A. 1961 Feb 15;47:137–163. doi: 10.1073/pnas.47.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S. Equilibrium sedimentation in density gradients of DNA preparations from animal tissues. J Mol Biol. 1961 Dec;3:711–716. doi: 10.1016/s0022-2836(61)80075-2. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]