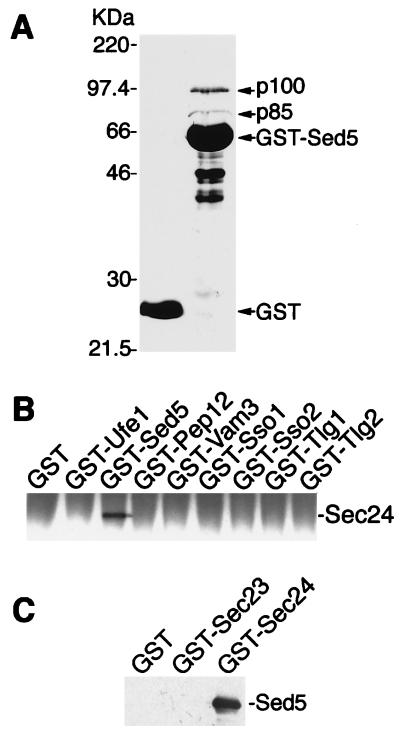
Figure 1.
Specific binding of Sec24p to the syntaxin-5 homologue Sed5p. (A) Proteins from detergent-lysed yeast cells were incubated at 4°C for 2 h with GST alone (lane 1) or GST-Sed5 (lane 2) immobilized on glutathione-Sepharose 4B. Beads were washed three times, and the bound proteins were analyzed by SDS/PAGE and Coomassie blue staining. p85 and p100 were identified as Sec23p and Sec24p, respectively, after elution from a preparative gel, endoproteinase Asp-N digestion, and peptide sequencing. The positions of molecular mass markers are indicated to the left. (B) Triton X-100-solubilized proteins (250 μg) from protease-deficient yeast cells were incubated in 200 μl lysis buffer with 1 μM GST or GST fusion proteins previously immobilized on glutathione-Sepharose beads. The bound proteins were fractionated by SDS/PAGE and detected by immunoblotting with a polyclonal anti-Sec24 antibody. (C) GST-Sec24, GST-Sec23, or GST (0.5 μM) was immobilized on glutathione-Sepharose and, after washing with 1 M KCl, was incubated with 60 μg of E. coli-soluble proteins containing His6-Sed5 lacking its membrane anchor. Proteins bound to beads were fractionated by SDS/PAGE, followed by immunoblotting with anti-Sed5 antibodies.