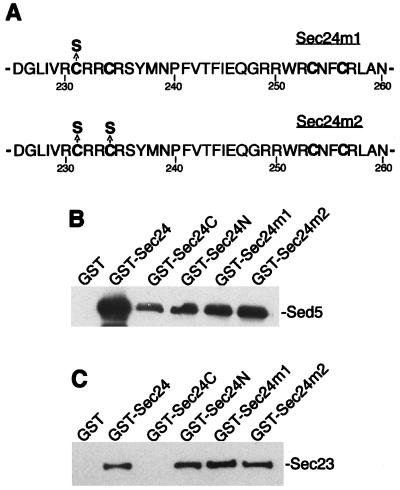
Figure 4.
Mutations in the putative zinc finger of Sec24p do not affect in vitro binding to Sed5p and Sec23p. The binding in vitro of Sed5p and Sec23p to GST and GST fusion proteins was performed as described in the legend to Fig. 1. (A) Amino acid substitutions in the putative zinc-finger of Sec24p. (B) Purified GST, GST-Sec24 (amino acids 1–926), GST-Sec24C (amino acids 550–926), GST-Sec24N (amino acids 56–549), GST-Sec24 m1 (amino acids 56–549), and GST-Sec24 m2 (56–549) (0.5 μM of each) were immobilized on glutathione-Sepharose beads and incubated with protein extract from E. coli expressing either His6-Sed5 (B) or His6-Sec23 (C). The bound proteins were separated by SDS/PAGE, followed by immunoblotting with antibody against Sed5p (B) or Sec23p (C).