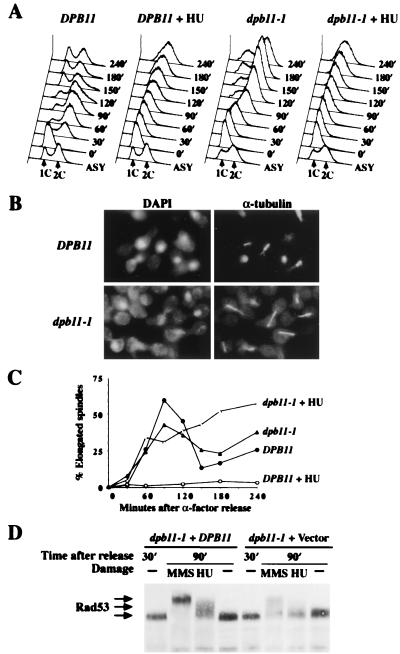
Figure 1.
The S-phase checkpoint is deficient in dpb11–1 mutant. (A–C) Wild-type (Y791) and dpb11–1 (Y792) cells were synchronized by α-factor at 24°C. After G1 arrest, cultures were shifted to 36°C for 45 min and divided into two, and equal portions were released from the block into SC-Ura or SC-Ura containing 0.2 M HU. At regular intervals after release, aliquots were withdrawn to examine DNA content, nuclear and spindle morphology. (A) FACS profile shows the DNA content of wild-type and dpb11–1 cells after release from G1 into medium with or without 0.2 M HU. Time indicates minutes after release. (Lower) Asynchronous cells untreated with HU at 24°C, which are included as a reference. (B) Photomicrographs of wild-type and dpb11–1 cells at 120 min after release from α-factor into SC-Ura with 0.2 M HU at 36°C. Nuclear morphology was visualized with 4′,6-diamidino-2-phenylindole, and microtubule morphology was visualized by indirect immunofluorescence using anti-α-tubulin antibody. (C) Kinetics of spindle elongation in the presence or absence of HU. (D) Y791 and Y792 were grown in SC-Ura, synchronized in G1 by α-factor, then prewarmed at 34°C for 1 hr before release at 34°C into media lacking α-factor. At 30 min after release, when most of the cells enter S phase, HU (0.2 M final) or MMS (0.1% final) was added to block DNA replication or damage DNA. An hour later (90 min after release), protein extracts were prepared, fractionated by SDS/PAGE, and immunoblotted with antibodies to Rad53 (2).