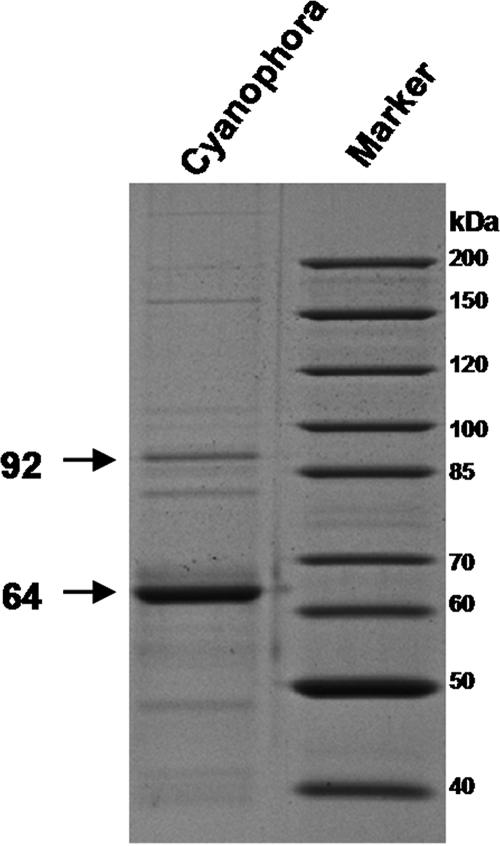
FIG. 4.
Visualization of the proteins associated with Cyanophora paradoxa starch granules. Granule-bound proteins were extracted by boiling them in sodium dodecyl sulfate (SDS) and were separated by SDS-PAGE (18). A molecular mass marker also is displayed. The major band at around 64 kDa was hydrolyzed with trypsin, and the resulting peptides were eluted from the gel and identified by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) and mass spectrometry-mass spectrometry (MS-MS) sequencing. The peptides YDQYFDAWDTSVR, VTFLLHNLLYQGR, DLPVNALATR, VFYETKGKDR, and SVPTTPLLAFVGR correspond to a GBSS and were found in the sequence isolated by cDNA screening. The band at around 92 kDa was hydrolyzed with trypsin, and the resulting peptides were eluted from the gel and identified by MALDI-TOF and MS-MS sequencing. The peptide MQAVQQRY corresponds to a starch phosphorylase and was found in recent Cyanophora paradoxa EST resources (GenBank accession no. EC661027) (35).