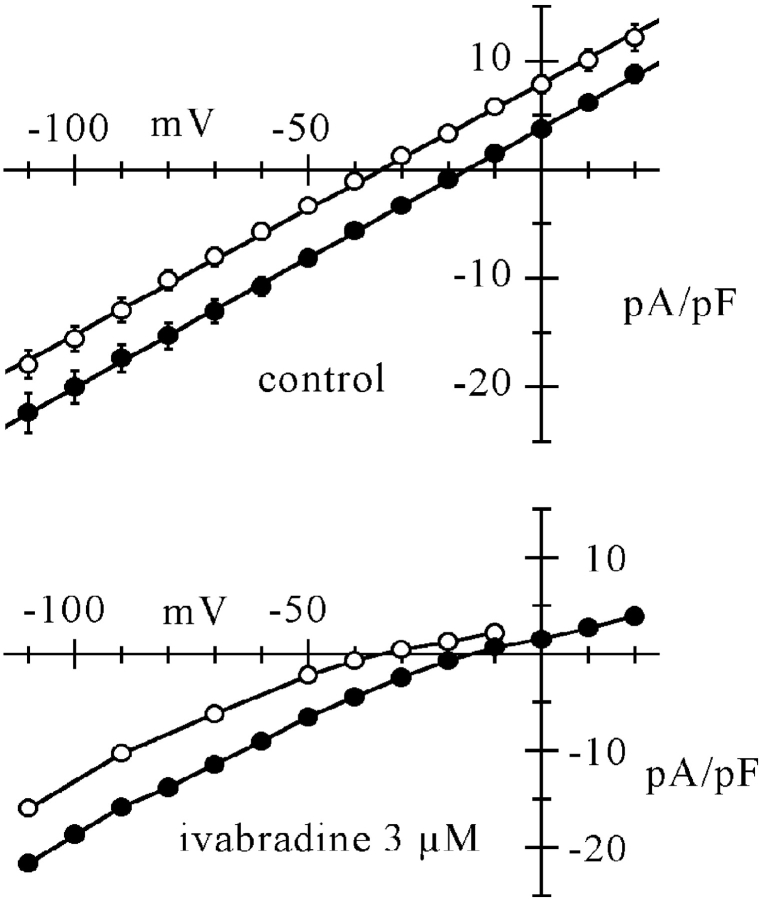
Figure 8.
Ivabradine causes inward rectification of If, which depends on E − Ef. Top: mean fully activated I/V relations measured from n = 7 cells exposed to both normal Tyrode solution (filled circles) and reduced (35 mM) Na+ concentration (open circles). I/V relations were measured as described previously (DiFrancesco et al., 1986), by applying pairs of steps, one to fully activate (1 s to −125 mV) and one to fully deactivate If (1 s to 15 mV), each followed by a step to the same test voltage, where the amplitude of the different current was measured. Mean ± SEM values are plotted. Linear fitting (straight lines) yielded reversal potentials (Ef) of −16.0 and −34.4 mV for normal and reduced Na+ concentration, respectively. Bottom: same curves, multiplied by fractional block values in normal and low Na+ solution as deduced from data in Fig. 7 at corresponding voltage and Na+ concentration. Strong rectification is apparent in the outward part of both curves, independently of the reversal potential. Lines through points.