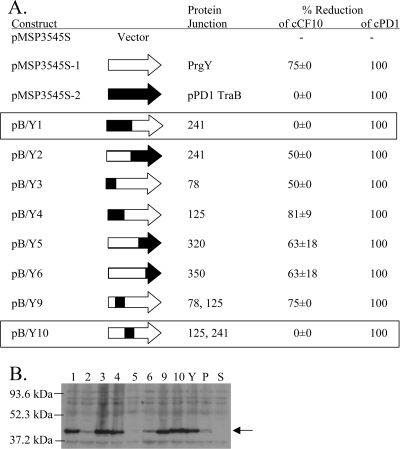
FIG. 6.
Specific reduction of cCF10 activity is conferred by PrgY amino acids 125 to 241. (A) cCF10 or cPD1 activity was measured in supernatants of TX5128 strains harboring the indicated construct. Cell culture supernatants (grown 6 h from a 10% overnight inoculum induced with 25 ng of nisin/ml) were diluted twofold, and the pheromone activity was determined in a clumping induction assay using OG1RF(pCF10) or (pPD1) indicator strains. The PrgY-dependent percent reduction of cCF10 activity averaged from two independent experiments (the error represents the standard deviation of two independent experiments; the cCF10 titer in the absence of PrgY, TraB, or a chimeric protein was 16 in the first experiment and 32 in the second). cPD1 was similarly assayed twice with the same results each time; the cPD1 titer was 4 in the absence of either PrgY or TraB, and all of the chimeras completely reduced all cPD1 activity. (B) The expression of pPD1 TraB (P), PrgY (Y) and TraB/PrgY chimeras 1 to 6, 9, and 10 (labeled 1 to 6, 9, and 10) in whole-cell extracts was analyzed by Western blotting with a polyclonal anti-PrgY antibody (8). TX5128 was the strain background in these experiments. The arrow at 43 kDa corresponds to the expected size of PrgY. All strains were treated with 25 ng of nisin/ml for 2.5 h prior to harvesting. An equivalent amount of protein was loaded for each sample.