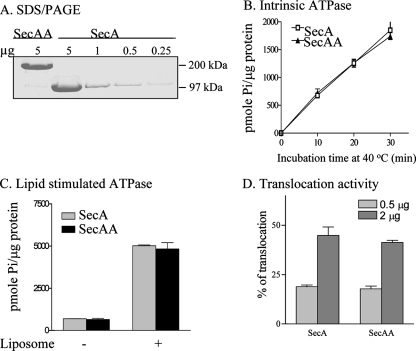
FIG. 4.
The isolated SecAA protein is functional in vitro. (A) SDS-PAGE and Coomassie blue staining of purified SecAA and SecA showing >97% purity. (B) Intrinsic ATPase activity of SecA and SecAA as a function of incubation time. Five micrograms each of purified SecA and SecAA was incubated in reaction mixtures at 40°C for the times indicated. (C) Lipid-stimulated ATPase activity of genetically linked SecA dimers. Two micrograms each of purified SecA and SecAA was incubated with 10 μg of liposomes at 30°C for 30 min. (D) In vitro protein translocation activity of 35S-Met-labeled pro-OmpA in the presence of the indicated amount of wild-type SecA or SecAA.