Figure 1.
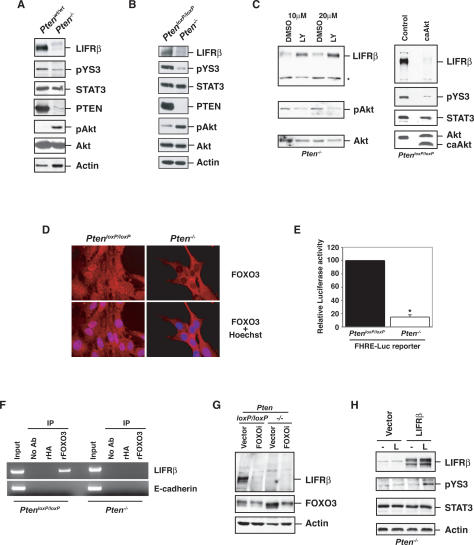
PTEN deficiency suppresses the LIFRβ–STAT3 signaling pathway. (A) Lysates of mouse Ptenwt/wt or Pten−/− astrocytes were immunoblotted with antibodies to LIFRβ, Tyr705-phosphorylated STAT3 (pYS3), total STAT3, PTEN, total Akt, Ser473-phosphorylated Akt (pAkt), or actin. (B) Immunoblotting of parental PtenloxP/loxP and Pten−/− astrocytes with antibodies used in A. Loss of PTEN expression was associated with an increase in phospho-Akt levels and with a decrease in LIFRβ and pYS3. Actin served as loading control. (C, left panel) Immunoblotting of lysates of Pten−/− astrocytes treated with the PI3K inhibitor LY294002 or DMSO vehicle control for 48 h. Asterisk indicates a nonspecific band. (Right panel) Immunoblotting of lysates from serum-starved PtenloxP/loxP astrocytes stably expressing a constitutively active form of Akt (caAkt). Activated Akt reduced LIFRβ and pYS3 levels. (D) Immunocytochemical analysis of PtenloxP/loxP and Pten−/− astrocytes with a FOXO3 antibody. Nuclei were stained with a DNA dye (Hoechst). FOXO3 was excluded from the nucleus in Pten−/− astrocytes. (E) PtenloxP/loxP and Pten−/− astrocytes were transfected with a luciferase reporter gene controlled by FOXO-binding sites (FHRE-Luc), together with a renilla expression plasmid to serve as an internal control, and subjected to a dual luciferase assay. FOXO-dependent transcription was reduced in Pten−/− astrocytes. (F) ChIP analysis at the endogenous LIFRβ promoter in PtenloxP/loxP and Pten−/− astrocytes with a FOXO3 antibody. A rabbit anti-HA antibody was used as negative control. The analysis was done with two independent sets of primers for the LIFRβ promoter. Negative controls for the PCR reaction were performed with primers for the E-cadherin promoter. Endogenous FOXO3 occupied the endogenous LIFRβ promoter in PtenloxP/loxP but not in Pten−/− astrocytes. (G) Immunoblotting with the LIFRβ and FOXO3 antibodies of PtenloxP/loxP and Pten−/− astrocytes infected with a FOXO3 RNAi-encoding lentivirus (FOXOi) or an empty vector and selected with puromycin. Actin served as loading control. Knockdown of endogenous FOXO3 reproducibly led to down-regulation of LIFRβ. (H) Immunoblotting of lysates of serum-starved Pten−/− astrocytes transfected with a LIFRβ expression plasmid or a control vector that were left untreated (−) or treated with LIF (L) for 15 min. LIFRβ restored LIF-induced STAT3 phosphorylation in Pten−/− astrocytes.