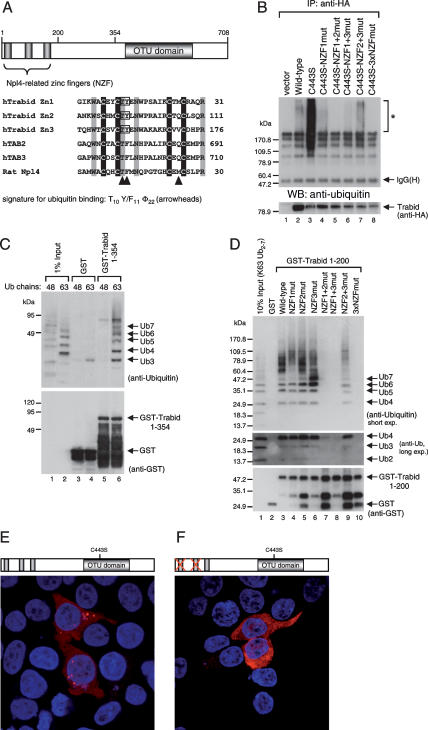
Figure 3.
Binding of Trabid’s NZF motifs to K63-linked ubiquitin. (A) Alignments of various NZF motifs, as indicated, with invariant cysteines shaded in black and other invariant residues in gray; the first cysteines (underlined) were mutated to alanine in the 3xCysNZF mutant. The T10 F/Y11 Φ22 signature for ubiquitin binding (Alam et al. 2004) is indicated by arrowheads (Φ, aliphatic amino acid; residue numbers refer to the position in this alignment); mutated TY residues are boxed. (B, Top) Ubiquitin-binding assays, with wild-type and mutant HA-Trabid immunoprecipitated from transfected 293T cells; asterisk indicates ubiquitylated protein coimmunoprecipitated with wild type and C443S; this signal is eliminated in the double-mutant C443S 3xNZFmut whose ubiquitin binding is abolished (see also D); background band, IgG heavy chain. (Below) The same blot was reprobed with α-HA antibody to monitor expression levels. (C) Ubiquitin-binding assays, with GST alone or GST-tagged Trabid(1–354) (see also Fig. 2C), expressed in bacteria (shown in bottom panel), and incubated in vitro with K48- or K63-linked ubiquitin; note the strong binding preference for K63-linked chains (shown in lanes 5,6). (D, top) Ubiquitin-binding assays as in C, with wild-type or mutant Trabid(1–200) (spanning the three tandem NZF motifs) incubated with K63-linked ubiquitin; TY > LV mutation of individual NZF (lanes 4–6), two NZF motifs (lanes 7–9), or all three NZF motifs (lane 10). (Middle panel) Long exposure of the same blot (bottom part only), to visualize residual binding of the NZF1 + 2 and NZF1 + 3 mutants to Ub4. (Bottom panel) The same blot was reprobed with α-GST antibody to monitor levels (revealing some breakdown; see also C). Note that the minimal module binding to K63-linked ubiquitin chains consists of at least two linked NZF motifs. (E,F) Immunofluorescence of 293 cells cotransfected with C443S and C443S-NZF1 + 2, fixed and stained as in Figure 1C–E.