Figure 1.
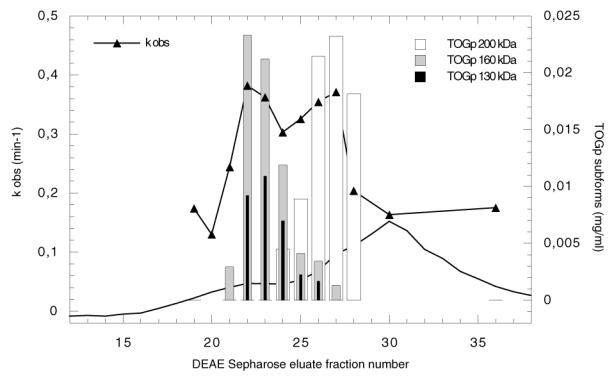
Purification of TOGp by ion exchange chromatography on DEAE Sepharose column. The experiment is performed in TEM buffer (Tris/HCl, 20 mM, pH 8.2, 1 mM EGTA, 1 mM Mg SO4, 1 μg/ml leupeptin). Proteins bound to the column are eluted with a 0 to 0.1 M KCl gradient in TEM buffer. The absorbance at 280 nm is indicated in arbitrary units (solid line). The apparent first order rate constant of microtubule elongation catalyzed by the eluate is determined as follows: a dialyzed sample (65 μl) of each fraction is incubated at 37°C with 20 μM tubulin in PEM buffer, 3.4 M glycerol, 5 mM Mg SO4, 1 mM GTP. The polymer formation is recorded at 350 nm for 30 min and the kobs expressed in min−1 is calculated from the kinetics. The concentration of the TOGp subforms is deduced from the densitometric analysis of the Western blots and from the total protein content of each chromatographic fraction. The difference in protein amount between fraction T22 and T20 is considered to represent the total amount of TOGp in fraction T22. This value is used to standardize the measure. The 130, 160 and 200 kDa TOGp subform concentrations are estimated in each fraction.
