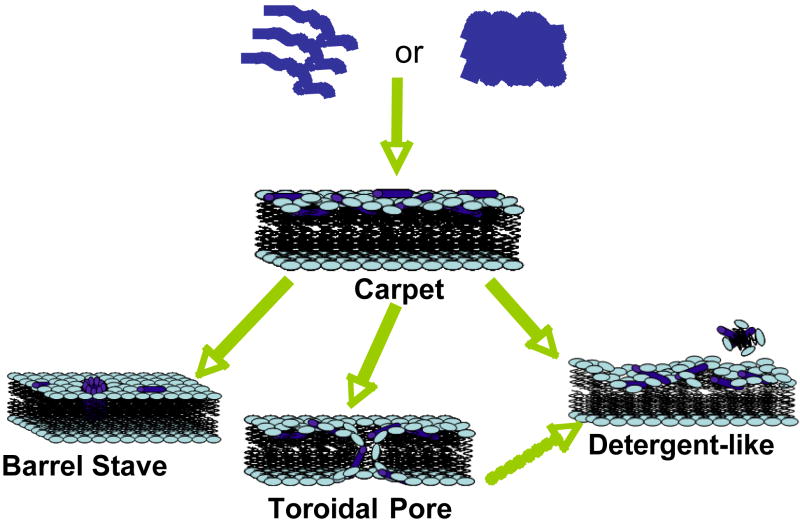
Figure 1. Models of AMP-induced membrane permeabilization.
Cationic linear antimicrobial peptides are initially unstructured monomers (most AMPs) or helical and aggregated (for example, LL-37) in aqueous solution. They bind to negatively charged membrane surface by electrostatic interactions. In the carpet model, peptides bind to phospholipid head groups and align themselves parallel to the membrane surface in a carpet-like fashion until a critical threshold concentration is reached. In the barrel stave model, peptides self aggregate in the membrane once a critical threshold concentration of peptide is reached, resulting in the formation of a transmembrane pore lined by peptide oriented such that the hydrophilic face forms the inner channel while the hydrophobic face is on the outside. Since an AMP has to span the entire thickness of the lipid bilayer in this model, a minimum of ~20 residues for α-helical peptide and ~8 residues for a β-sheet peptide is required to function via the barrel stave mechanism. The toroidal pore model is an extension of the barrel stave model and postulates that at some critical concentration of peptide curvature strain induces the membranes to curve inward, resulting in the formation of a pore that is lined by both peptide and lipid headgroups. Repulsive interactions between the positively charged residues of the peptide are minimized due to the presence of the negatively charged phospholipids in the pore-lining. While the formation of these pores depend on the lipid:peptide ration and the ionic selectivity depend on the membrane composition, the lifetime of these pores seem to vary. The detergent-like model proposes that peptides intercalate in between the phospholipid head groups in a cone-like fashion causing curvature strain and micellization at local regions of high peptide density or when preformed peptide aggregates interact with lipid membranes. Recent NMR studies suggested that after sufficient time (may be a month or longer) AMPs fragment the lipid bilayers (even those containing toroidal pores) to form bicelle or micelle-like structures. Other membrane-disruptive and non-membrane disruptive mechanisms of some AMPs are discussed in the text.