Figure 6.
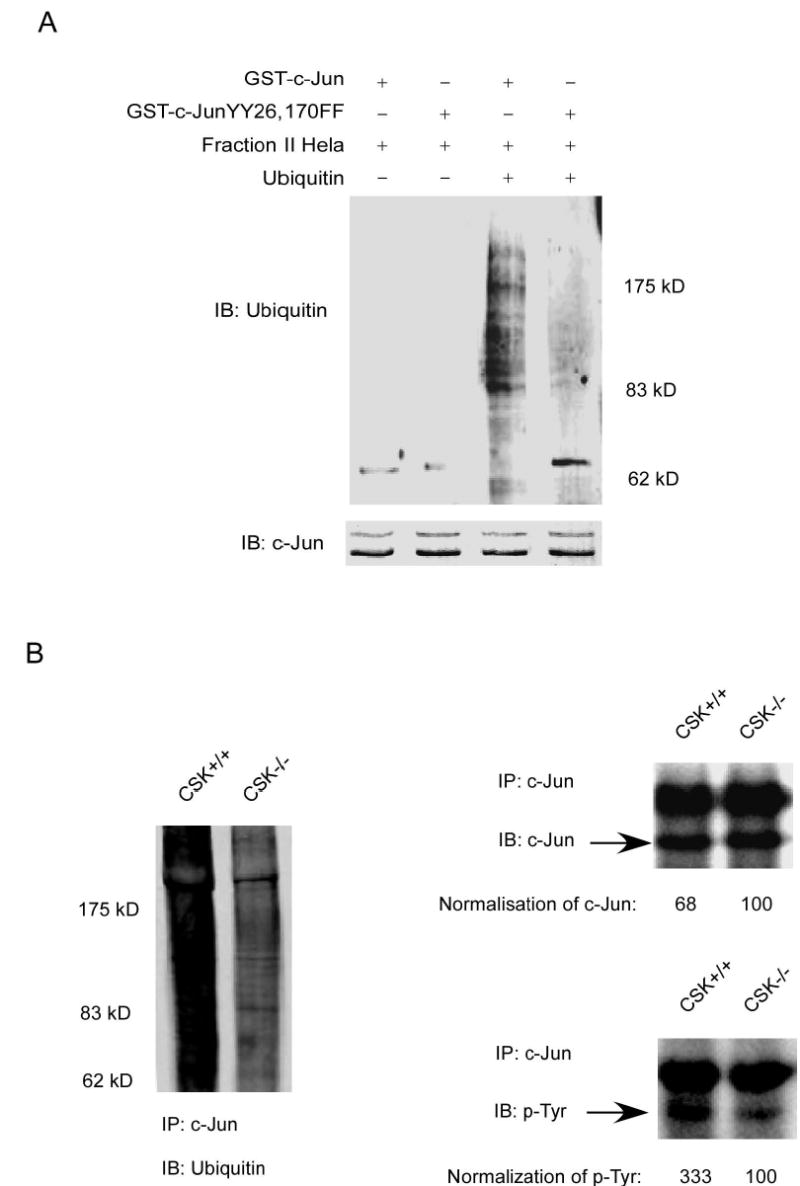
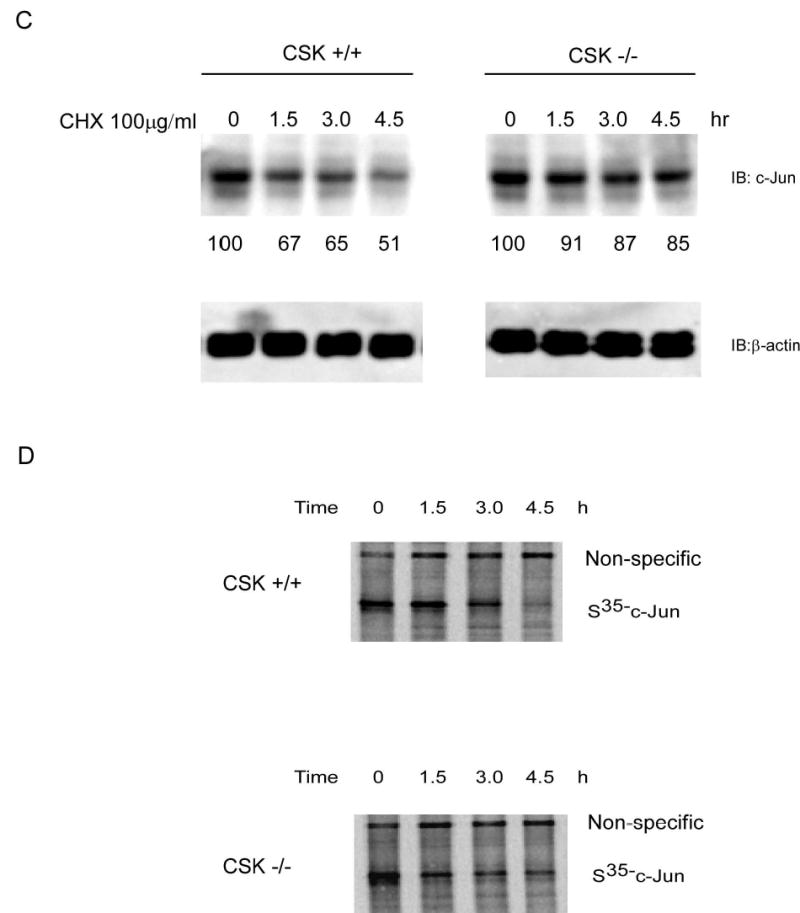
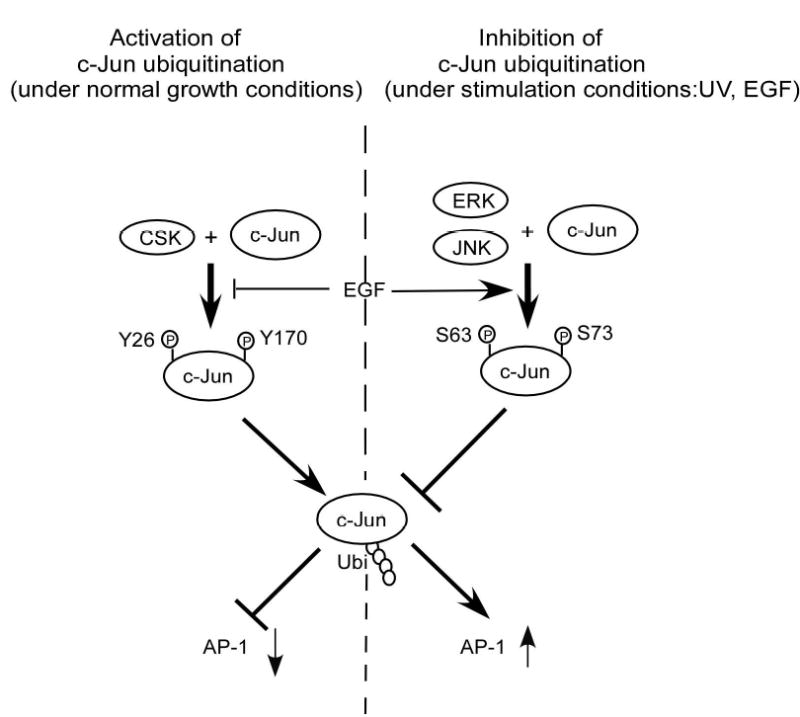
CSK-mediated c-Jun phosphorylation promotes c-Jun degradation. A, in vitro ubiquitination assay for GST wild type c-Jun or GST c-Jun double mutant (YY26, 170FF). B, the level of phosphorylated tyrosine was much higher in CSK+/+ cells than in CSK−/− cells and the total amount of c-Jun protein was also higher in CSK−/− cells than in CSK+/+ cells. Ubiquitination of c-Jun was stronger in CSK+/+ cells than in CSK−/− cells. C, stability of c-Jun was analyzed in CSK+/+ cells or CSK−/− cells at indicated time points by immunoblotting after addition of cycloheximide. D, stability of c-Jun in CSK+/+ cells or CSK−/− cells was analyzed by pulse-chase experiments. E, schematic illustration of the regulation of c-Jun ubiquitination. Under normal growth conditions, CSK phosphorylates c-Jun, which promotes ubiquitin-dependent degradation of c-Jun, resulting in a decrease in AP-1 activity. In contrast, with EGF stimulation, CSK is inhibited and c-Jun is activated by phosphorylation by MAP kinases, which inhibits ubiquitin-dependent degradation of c-Jun, resulting in an increased AP-1 activity.
