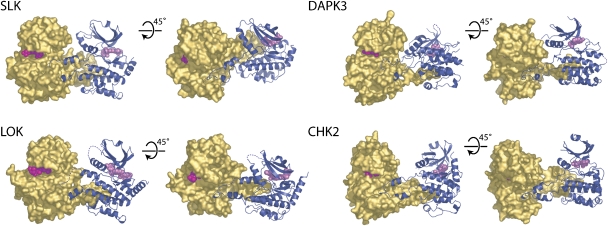
Figure 2.
Domain-exchanged kinase dimers. The activation segment regions from each monomer extend to form an extensive intermolecular interface. The arrangements of monomers within each dimer are distinct and the tip of each activation segment binds in a narrow cleft formed between helix αF and αG in the C-terminal lobe of the kinase. For each dimer, two views are shown separated by a 45° and a molecular surface (gold) is shown for one monomer, while the other monomer is shown in ribbon form (dark blue). Disordered regions are shown as dotted lines and bound inhibitors are depicted in space-filling form. Each dimer is shown in the same reference frame as that of SLK.