Abstract
Background
Tetracyclines are used in periodontal therapy as antimicrobial agents and as inhibitors of matrix metalloproteinases. Neutrophils appear to accumulate minocycline and other tetracyclines through a mechanism that has not been fully characterized.
Methods
The transport of minocycline and other tetracyclines by isolated human neutrophils was characterized by measuring the increase in cell-associated fluorescence.
Results
Quiescent neutrophils took up minocycline through a saturable, concentrative, sodium-dependent mechanism with a Michaelis constant (Km) of 153 μg/ml (501 μM) and a maximal velocity of 240 ng/minute/106 cells. The efficiency of minocycline transport was not influenced significantly by a two-unit variation in extracellular pH and was not enhanced upon cell activation with phorbol myristate acetate. Neutrophil incubation in medium containing 10 μg/ml minocycline, doxycycline, or tetracycline yielded steady-state intracellular/extracellular concentration ratios of ∼64.0, 7.5, or 1.8, respectively. The dilution of extracellular minocycline or doxycycline triggered efflux from cells loaded with these antibiotics. Minocycline transport was competitively inhibited by the organic cations carnitine, diphenhydramine, and verapamil, but penicillin and other organic anions failed to produce inhibition.
Conclusion
Transport of tetracyclines by neutrophils could potentially enhance the effectiveness of these agents in periodontal therapy by enhancing or sustaining their therapeutic levels at inflammatory sites and by enhancing the killing of phagocytosed bacterial pathogens.
Keywords: Drug delivery, infection, inflammation, periodontitis, phagocyte, tetracycline
Tetracyclines are a class of antimicrobial agents that inhibit ribosomal protein synthesis1 and produce bacteriostatic effects against an unusually broad spectrum of bacteria. They have been widely used as adjuncts in the treatment of aggressive and recurrent forms of periodontitis due to their ability to inhibit Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and many other periodontal microorganisms at concentrations attainable in vivo.2,3 In addition, tetracyclines have been used at subantimicrobial doses in dental practice to inhibit neutrophil collagenase and other matrix metalloproteinases.4,5 In contrast to many widely used antimicrobial agents (e.g., cephalosporins and β-lactam antibiotics), there is evidence that tetracyclines accumulate inside phagocytes.6-8 This process could potentially contribute to the enhanced intracellular killing of bacteria and decreased activity of neutrophil-derived matrix metalloproteinases relative to control neutrophils that contain no antimicrobial agent. If neutrophils could carry tetracyclines with them as they migrate to infection sites, they could potentially enhance the local tetracycline concentration at these sites. Because neutrophils infiltrate these sites in great numbers, this could result in enhanced resolution of tetracycline-susceptible infections.
Little is known about the mechanisms by which neutrophils take up tetracyclines. A previous study showed that uptake is temperature dependent,7 but it is unclear whether the process occurs by active transport or facilitated diffusion. Our previous work demonstrated that neutrophils take up fluoroquinolone antimicrobial compounds by a transport mechanism that is energy dependent, obeys Michaelis-Menten kinetics, and is strongly upregulated by agents that activate protein kinase C.9,10 Many cationic and zwitterionic drugs are actively transported by the widely distributed family of organic cation transporters. Because tetracyclines possess amine groups that could potentially interact with these transporters, we hypothesized that organic cation transporters play a role in the active transport of tetracyclines into neutrophils. This article provides evidence to support this hypothesis.
MATERIALS AND METHODS
Neutrophil Isolation
Volunteer blood donors were recruited between May 2000 and August 2001 under a protocol approved by the University’s Institutional Review Board. The donor population ranged from 20 to 49 years of age and included five males and six females. Human neutrophils were isolated from citrated whole blood obtained from healthy volunteers using Ficoll-Hypaque density gradient centrifugation and dextran sedimentation.11 Residual erythrocytes were eliminated by hypotonic lysis. The remaining neutrophils were washed three times with phosphate buffered saline. For the assays described below, neutrophils (typically >98% pure and >98% viable) were resuspended in modified Hank’s balanced salt solution (HBSS) comprising 1.9 mM KH2PO4, 1.1 mM Na2HPO4, 5 mM KCl, 147 mM NaCl, 5.5 mM glucose, 1 mMMgCl2, and 1 mMCaCl2, pH7.3.
Transport Assays
The transport of minocycline and other tetracyclines was assayed by measuring cell-associated fluorescence as previously described.12 Neutrophils were suspended in HBSS at a density of 5 × 106 cells/ml and warmed to 37°C prior to incubation with the indicated tetracycline. The assay was terminated by withdrawing aliquots of cell suspension, layering the suspension over 0.3 ml canola oil/dibutyl phthalate (3:10), and centrifuging for 45 seconds at 15,000 × g in a microcentrifuge. The aqueous and oil layers were removed, and the cell pellet was recovered by cutting off the end of the microcentrifuge tube. The pellet was dispersed and lysed in 1 ml water by agitation at room temperature. The samples were clarified by centrifugation at 5,600 × g for 5 minutes. For measurement of minocycline, the lysate was added to an equal volume of ethylene glycol containing 200 mM citric acid and 200 mM magnesium acetate and analyzed by fluorescence spectroscopy.13 To determine the affinity and velocity of transport, the kinetics of transport were measured in the presence of several minocycline concentrations (10 to 154 μg/ml) during the linear initial phase (0 to 3 minutes) and analyzed by the Lineweaver-Burk method. The Michaelis constant (Km) and maximum transport velocity (Vmax) values were derived from regression lines obtained with the plotted data.
Intracellular Volume Measurements
To calculate intracellular antibiotic concentrations, intracellular content was divided by intracellular volume. The latter was measured by equilibrating neutrophil suspensions with (3H)-water† (5 μCi/ml).14 After 10 minutes, cells were rapidly pelleted through oil as previously described. The pellet was lysed and counted with a liquid scintillation system. To correct for the extracellular water trapped in the pellet, cells were equilibrated with (14C)-inulin‡ (2 μCi/ml) and processed similarly.
RESULTS
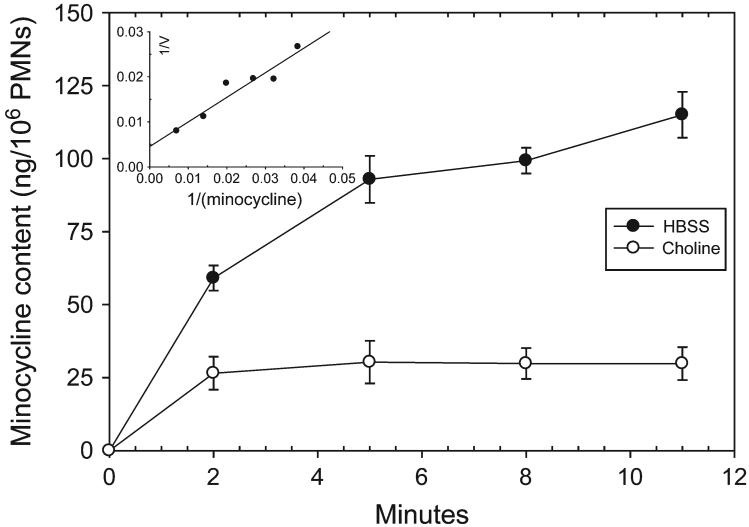
Human neutrophils took up minocycline from the extracellular medium in a saturable manner (Fig. 1). Neutrophil minocycline accumulation was significantly inhibited when Na+ was eliminated from the assay buffer by substitution of K2HPO4 for Na2HPO4 and choline chloride for NaCl (Fig. 1). The kinetics of uptake yielded linear plots when analyzed by the Lineweaver-Burk method (Fig. 1, inset). These assays suggested the involvement of a transporter with a Km of 152 ± 12.3 μg/ml and a Vmax of 240 ± 17.2 ng/minute/106 cells. Despite the relatively low affinity of transport, neutrophils accumulated relatively high intracellular minocycline levels. Incubation at 37°C with 10 μg/ml minocycline yielded a steady-state intracellular concentration that was ∼64-fold higher than the extracellular level (Table 1). Other tetracyclines were also concentrated by neutrophils. Incubation with 10 μg/ml doxycycline or tetracycline resulted in steady-state intracellular concentrations that were 7.5- and 1.8-fold higher, respectively.
Figure 1.
The sodium dependence of minocycline transport by human neutrophils. Neutrophils were suspended in HBSS containing NaCl or a Na+-free balanced salts solution containing choline chloride. Minocycline (10 μg/ml) was added and uptake was assayed at 37°C. The data are expressed as the mean (±SEM) transport activity of three experiments. Significant differences in intracellular minocycline content were observed at every time point (P ≤0.01; t test). The insert shows the regression line from Lineweaver-Burk analysis of minocycline transport kinetics (r = 0.98).
Table 1.
Cellular/Extracellular Concentration Ratios Observed in Resting Human Polymorphonuclear Leukocytes
| Extracellular Concentration (μg/ml) | Cellular/Extracellular for Minocycline | Cellular/Extracellular for Doxycycline | Cellular/Extracellular for Tetracycline |
|---|---|---|---|
| 2 | 84.7 ± 8.9 | 8.06 ± 5.60 | 14.0 ± 1.4* |
| 5 | 65.5 ± 3.9 | 6.39 ± 1.46 | 5.49 ± 0.69 |
| 10 | 64.2 ± 6.7 | 7.55 ± 1.24 | 1.81 ± 0.49 |
| 20 | 60.6 ± 6.6 | 9.84 ± 1.34 | 2.72 ± 0.61 |
Results are expressed as the mean of four determinations ± SEM. Within each column, the differences in the mean values are greater than would be expected by chance (P <0.05; ANOVA).
Values that are significantly different from other means in the same column (P <0.05; Tukey test).
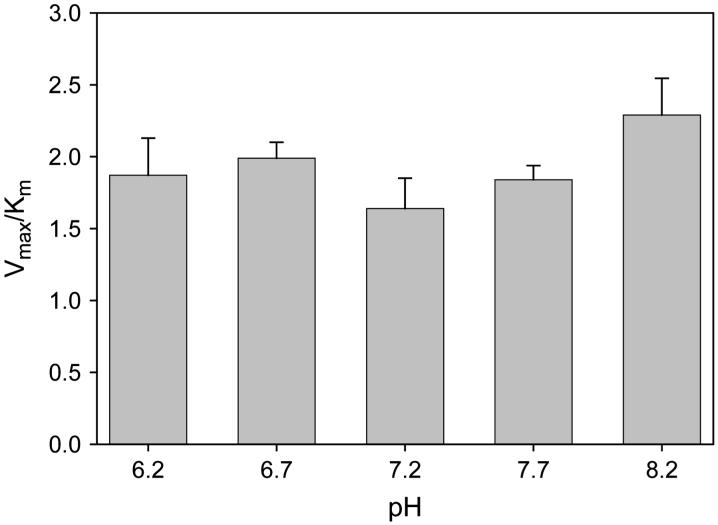
To determine the effect of pH on neutrophil minocycline transport, uptake kinetics were analyzed over the range of pH 6.2 to 8.2 (Fig. 2). Within this range, changes in pH did not significantly alter the efficiency of transport (as assessed by Vmax/Km ratio) (P >0.3; analysis of variance [ANOVA]).
Figure 2.
The pH dependence of minocycline transport by quiescent neutrophils. Cells were suspended in HBSS adjusted to the indicated pH, and minocycline transport was assayed at 37°C. Data are presented as the mean ± SEM of three experiments. The transport was not significantly influenced by pH (P = 0.34; ANOVA).
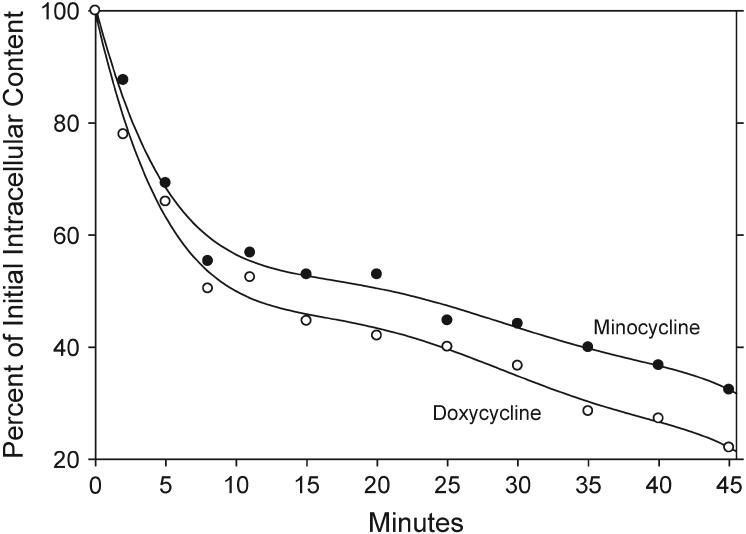
Neutrophils loaded with minocycline or doxycycline lost most of their antibiotic content within minutes after their concentrations in the extracellular medium were diluted (Fig. 3). Under these conditions, half of the minocycline content was lost within ∼22 minutes, and half of the doxycycline content was lost within 11 minutes. After 40 minutes, neutrophils retained 37% of their minocycline content and 27% or their doxycycline content.
Figure 3.
Efflux of minocycline and doxycycline from loaded neutrophils. Cells were loaded to a steady-state intracellular concentration by incubation for 15 minutes at 37°C in HBSS containing 30 μg/ml minocycline or doxycycline. Efflux was triggered by diluting the extracellular antimicrobial solutions 1:20 with 37°C HBSS and monitored by the decrease in cell-associated fluorescence.
Inhibition studies were performed to determine whether minocycline transport could be inhibited with other organic cations. Kinetic analysis revealed that carnitine, diphenhydramine, and verapamil acted as competitive inhibitors of minocycline transport, with inhibition constants (Ki values) of 2.2 ± 0.67 mM, 1.3 ± 0.19 mM, and 1.01 ± 0.16 mM, respectively (data not shown). The organic anions penicillin, cephalothin, prednisolone, and hydrocortisone failed to produce a significant inhibition of minocycline transport.
DISCUSSION
Neutrophils express a saturable, sodium-dependent transport system that allows them to take up and accumulate minocycline and other tetracyclines. This transport system exhibits Michaelis-Menten kinetics and a relatively low affinity for substrate. Compared to the kinetics of fluoroquinolone transport by neutrophils, minocycline is transported with a similar affinity but at a higher velocity.9 Despite the low affinity, neutrophils can accumulate high intracellular concentrations of tetracyclines. The observed cellular/extracellular concentration (C/E) ratios were >60 for minocycline and >7 for doxycycline. These experiments were conducted with antibiotic concentrations similar to those found in vivo (1 to 4 μg/ml in serum and 2 to 12 μg/ml in gingival crevicular fluid).15,16 Clinical trials with locally delivered sustained-release tetracycline agents have yielded gingival crevicular fluid concentrations that are at least 100-fold higher than those attainable with a systemic route of administration.
The ability to accumulate tetracyclines is not a unique characteristic of neutrophils. In previous studies,12,17 gingival fibroblasts and oral epithelial cells that were incubated with minocycline under similar conditions attained C/E ratios of >60 and >50, respectively. It seems reasonable to predict that these C/E ratios are lower in vivo, because a portion of minocycline in the serum and interstitial fluid is bound to protein. Nevertheless, these elevated intracellular concentrations could potentially enhance the phagocytic killing of susceptible pathogens by neutrophils. Previous work18 has shown the ciprofloxacin-loaded neutrophils killed significantly more A. actinomycetemcomitans and achieved significantly shorter half times for killing than control neutrophils. This effect was most pronounced when the bacteria-to-neutrophil ratio was relatively high.
The characteristics of the minocycline transport system were probed by identifying compounds that competitively inhibit minocycline uptake. Tetracyclines possess primary and tertiary amine groups, making them potential substrates for organic cation transporters. Organic cation transporters interact with a variety of cationic and zwitterionic compounds and are widely expressed in human tissues.19,20 In the present study, minocycline transport was competitively inhibited by the organic cations carnitine, diphenhydramine, and verapamil. Penicillin, cephalothin, and prednisolone, which are known to interact with organic anion transporters, produced no significant inhibition of minocycline transport. This suggests that neutrophils accumulate minocycline through a transport system that interacts with classical organic cations. The observed differences in uptake between minocycline, doxycycline, and tetracycline are presumably related to differences in the functional groups attached to the four-ringed structure of these compounds.
Neutrophil minocycline transport does not appear to be strongly influenced by a variation of one unit above or below physiological pH. This range lies below the pKa of the amine groups at positions 4 and 7 in minocycline and above the pKa of the hydroxyl group at position 3, so variation within this range should not significantly affect ionization of these functional groups. This finding may have clinical relevance because previous studies have reported that inflamed periodontal pockets are more alkaline than healthy gingival crevices.21,22 Thus, neutrophils could retain their ability to accumulate tetracyclines after migrating into the mildly acidic environment found in healthy gingival crevices or the slightly alkaline environment associated with periodontal pockets.
Most transporters are capable of moving substrate in a forward or reverse direction across the plasma membrane to maintain equilibrium between intracellular and extracellular substrate concentrations. Consistent with this, neutrophils loaded with minocycline or doxycycline can be induced to release these antibiotics by diluting their concentrations in the extracellular medium (Fig. 3). Forward transport could result in the enhanced killing of bacteria phagocytosed by neutrophils. Reverse transport could potentially play a role in maintaining effective tetracycline levels at inflammatory sites after levels in the blood serum return to baseline. Overall, the tetracycline transport system of neutrophils could have a favorable influence on the antimicrobial and anti-MMP effects of tetracyclines used in the treatment of periodontal disease. Additional studies are warranted to determine whether this system can be modulated or exploited in a manner that enhances these potentially beneficial effects.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant DE12601 from the National Institute of Dental and Craniofacial Research.
Footnotes
NEN Life Science Products, Boston, MA.
NEN Life Science Products.
REFERENCES
- 1.Goldman RA, Hasan T, Hall CC, Strycharz WA, Cooperman BS. Photoincorporation of tetracycline into Escherichia coli ribosomes. Identification of the major proteins photolabeled by native tetracycline and tetracycline photoproducts and implications for the inhibitory action of tetracycline on protein synthesis. Biochemistry. 1983;22:359–368. doi: 10.1021/bi00271a020. [DOI] [PubMed] [Google Scholar]
- 2.Walker CB, Gordon JM, McQuilkin SJ, Niebloom TA, Socransky SS. Tetracycline: Levels achievable in gingival crevice fluid and in vitro effects on subgingival organisms. II. Susceptibilities of periodontal bacteria. J Periodontol. 1981;52:613–616. doi: 10.1902/jop.1981.52.10.613. [DOI] [PubMed] [Google Scholar]
- 3.Walker CB, Pappas JD, Tyler KZ, Cohen S, Gordon JM. Antibiotic susceptibilities of periodontal bacteria. In vitro susceptibilities to eight antimicrobial agents. J Periodontol. 1985;56(Suppl):67–74. doi: 10.1902/jop.1985.56.11s.67. [DOI] [PubMed] [Google Scholar]
- 4.Golub LM, Goodson JM, Lee HM, Vidal AM, McNamara TF, Ramamurthy NS. Tetracyclines inhibit tissue collagenases. J Periodontol. 1985;56(Suppl):93–97. doi: 10.1902/jop.1985.56.11s.93. [DOI] [PubMed] [Google Scholar]
- 5.Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71:521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- 6.Park JK, Dow RC. The uptake and localization of tetracycline in human blood cells. Br J Exp Pathol. 1970;51:179–182. [PMC free article] [PubMed] [Google Scholar]
- 7.Laufen H, Wildfeuer A. Kinetics of the uptake of antimicrobial agents by human polymorphonuclear leukocytes. Arzneimittelforschung. 1989;39:233–235. [PubMed] [Google Scholar]
- 8.Gabler WL. Fluxes and accumulation of tetracyclines by human blood cells. Res Commun Chem Pathol Pharmacol. 1991;72:39–51. [PubMed] [Google Scholar]
- 9.Walters JD, Zhang F, Nakkula RJ. Mechanisms of fluoroquinolone transport by human neutrophils. Antimicrob Agents Chemother. 1999;43:2710–2715. doi: 10.1128/aac.43.11.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walters JD, Nakkula RJ. Ciprofloxacin transport by chemoattractant-activated polymorphonuclear leukocytes: Regulation by priming and protein kinase C. Antimicrob Agents Chemother. 2003;47:3345–3348. doi: 10.1128/AAC.47.10.3345-3348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 12.Yang Q, Nakkula RJ, Walters JD. Accumulation of ciprofloxacin and minocycline by cultured human gingival fibroblasts. J Dent Res. 2002;81:836–840. doi: 10.1177/154405910208101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lever M. Improved fluorometric determination of tetracyclines. Biochem Med. 1972;6:216–222. doi: 10.1016/0006-2944(72)90041-5. [DOI] [PubMed] [Google Scholar]
- 14.Garraffo R, Jambou D, Chichmanian RM, Ravoire S, Lapalus P. In vitro and in vivo ciprofloxacin pharmakinetics in human neutrophils. Antimicrob Agents Chemother. 1991;35:2215–2218. doi: 10.1128/aac.35.11.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Winkelhoff AJ, Rams TE, Slots J. Systemic antibiotics in periodontics. Periodontol. 2000;199610:45–78. doi: 10.1111/j.1600-0757.1996.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 16.Lavda M, Clausnitzer CE, Walters JD. Distribution of systemic ciprofloxacin and doxycycline to gingiva and gingival crevicular fluid. J Periodontol. 2004;75:1663–1667. doi: 10.1902/jop.2004.75.12.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brayton JJ, Yang Q, Nakkula RJ, Walters JD. An in vitro model of ciprofloxacin and minocycline transport by oral epithelial cells. J Periodontol. 2002;73:1267–1272. doi: 10.1902/jop.2002.73.11.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cacchillo DA, Walters JD. Effect of ciprofloxacin on killing of Actinobacillus actinomycetemcomitans by polymorphonuclear leukocytes. Antimicrob Agents Chemother. 2002;46:1980–1984. doi: 10.1128/AAC.46.6.1980-1984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koepsell H. Organic cation transporters in intestine, kidney, liver and brain. Annu Rev Physiol. 1998;60:243–266. doi: 10.1146/annurev.physiol.60.1.243. [DOI] [PubMed] [Google Scholar]
- 20.Dresser MJ, Zhang L, Giacomini KM. Molecular and functional characteristics of cloned human organic cation transporters. Pharm Biotechnol. 1999;12:441–469. doi: 10.1007/0-306-46812-3_15. [DOI] [PubMed] [Google Scholar]
- 21.Kleinberg I, Hall G. pH and depth of gingival crevices in different areas of the mouth of fasting humans. J Periodontal Res. 1969;4:109–117. doi: 10.1111/j.1600-0765.1969.tb01955.x. [DOI] [PubMed] [Google Scholar]
- 22.Bickel M, Cimasoni G. The pH of human crevicular fluid measured by a new microanalytical technique. J Periodontal Res. 1985;20:35–40. doi: 10.1111/j.1600-0765.1985.tb00408.x. [DOI] [PubMed] [Google Scholar]