Abstract
Gingival fibroblasts actively accumulate tetracyclines, thereby enhancing their redistribution from blood to gingiva. Since growth factors and pro-inflammatory cytokines regulate many fibroblast activities, they could potentially enhance fibroblast minocycline accumulation. To test this hypothesis, we treated gingival fibroblast monolayers for 1 or 6 hours with platelet-derived growth factor-BB (PDGF), fibroblast growth factor-2 (FGF), transforming growth factor-β1 (TGF), or tumor necrosis factor-α (TNF). Minocycline uptake was assayed at 37° by a fluorescence method. All 4 factors significantly enhanced minocycline uptake (P ≤ 0.008, ANOVA), primarily by increasing the affinity of transport. Treatment for 6 hours with 10 ng/mL FGF, PDGF, TGF, or TNF enhanced fibroblast minocycline uptake by 19% to 25%. Phorbol myristate acetate enhanced fibroblast minocycline uptake by 28%, suggesting that protein kinase C plays a role in up-regulating transport. These effects on transport provide a mechanism by which systemic tetracyclines could be preferentially distributed to gingival wound or inflammatory sites.
Keywords: tetracyclines, antimicrobial chemotherapy, aggressive periodontitis, matrix metalloproteinases
INTRODUCTION
Aggressive forms of periodontitis are difficult to treat by conventional scaling and root planing alone, because some of the associated bacterial pathogens (e.g., Actinobacillus actinomycetemcomitans) are capable of invading epithelial cells lining the pocket or gingival crevice (Christersson et al., 1987; Fives-Taylor et al., 1996). For this reason, tetracycline antibiotics are frequently used as adjuncts in the treatment of aggressive periodontitis. Tetracyclines can penetrate epithelial cells and are capable of inhibiting A. actinomycetemcomitans (Pavicic et al., 1992; van Winkelhoff et al., 1996). Interestingly, they appear to distribute preferentially to gingival crevicular fluid (GCF). When taken by the systemic route, tetracyclines attain GCF levels that are approximately 4- to 5-fold higher than serum levels (Ciancio et al., 1980; Pascale et al., 1986). Our recent work has shown that gingival fibroblasts express transporters that allow them to take up extracellular tetracyclines and sequester them in the gingiva (Yang et al., 2002). Although the affinity of transport is relatively low, fibroblasts accumulate remarkable amounts of these agents. For minocycline, the observed cellular/extracellular concentration (C/E) ratios were > 60. These findings suggest that gingival fibroblasts can function as reservoirs for tetracyclines. Their ability to accumulate high levels of these agents may enhance redistribution of tetracyclines from the bloodstream to the gingiva and sustain their therapeutic levels in GCF.
In the periodontium, PDGF, FGF-2, and TGF-β1 serve as important biological mediators for wound healing and regeneration (Cochran and Wozney, 1999), while TNF-α and IL-1β coordinate the host response and play a role in pathogenesis of tissue destruction associated with periodontitis (Birkedal-Hansen, 1993; Graves and Cochran, 2003). These growth factors and pro-inflammatory cytokines regulate fibroblast proliferation, adhesion, migration, and matrix synthesis (Clark, 1996). If fibroblast tetracycline transport were up-regulated by these factors, it could enhance the distribution of antimicrobial agents to the gingiva. In this study, we examined the effects of several growth factors and pro-inflammatory cytokines on minocycline accumulation by cultured fibroblasts. We used minocycline as a proxy for other tetracyclines, because its intense fluorescence allows transport to be easily assayed.
MATERIALS & METHODS
Isolation and Culture of Gingival Fibroblasts
Fibroblasts were isolated from an explant obtained from a healthy (firm, non-edematous, non-bleeding, pink) interproximal papilla of a 36-year-old male as previously described (Mariotti and Cochran, 1990), according to an IRB-approved informed consent procedure. Cells were cultured at 37°C in 5% CO2 in minimal essential medium (MEM, Life Technologies Inc., Rockville, MD, USA) supplemented with 2 mM glutamine and 10% heat-inactivated fetal bovine serum. For the experiments described below, 3.2 × 104 fibroblasts were seeded into each well of a 24-well cell culture plate. The cells were fed 3 days later and formed a confluent monolayer (as assessed by microscopy) within 6 days. Individual experiments were designed to have triplicate wells of each experimental condition. To enhance fibroblast responsiveness to growth factors and cytokines, confluent cells were starved for 20 hrs in MEM containing 0.5% fetal bovine serum prior to treatment with these mediators. Fibroblasts were used between passage numbers 4 and 15.
Conditions for fibroblast treatment with medium alone (control), PDGF-BB, FGF-2 (basic FGF), TGF-β1, TNF-α, IL-1β, or phorbol myristate acetate (PMA) are specified in the Fig. legends and Table footnotes. The range of treatment concentrations was adjusted for each agent, so the highest concentration corresponded to the dose that produced the maximum response observed in vitro. Growth factors and cytokines were purchased from PeproTech (Princeton, NJ, USA). Serum and all other biochemicals were purchased from Sigma Chemical Co (St. Louis, MO, USA).
Assay of Minocycline Transport
Transport was assayed by the measurement of cell-associated minocycline fluorescence. Multiwell culture plates containing confluent cell monolayers were washed 4 times with Hanks’ balanced salts solution (HBSS), overlaid with 0.2 mL/well HBSS, and warmed to 37°C prior to assay. The assay was initiated by the addition of 0.2 mL of warm HBSS containing twice the desired final minocycline concentration to each well, by means of multichannel pipettes. After incubation at 37°C for 3 min, the minocycline solutions were quickly removed. Each well was rapidly washed 4 times with HBSS to eliminate extracellular antibiotics, according to the method described by Gazzola et al. (1981). Cell monolayers underwent lysis in 1 mL of water. The lysate was centrifuged at 13,000 x g for 6 min and mixed with an equal volume of ethylene glycol containing 200 mM citric acid and 200 mM magnesium acetate. Fluorescence of the mixture was measured as previously described (Lever, 1972). Antibiotic content was normalized to cell protein by the method of Bradford (1976).
The rate of fibroblast minocycline transport is constant for approximately 3 minutes after cell exposure to the antibiotic in vitro. To determine the affinity and velocity of transport, we measured the kinetics of transport during this linear initial phase and analyzed it by the Lineweaver-Burk method. We used EnzPack for Windows (Biosoft, Ferguson, MO, USA) to derive the Michaelis constant (Km) and maximum transport velocity (Vmax) values from regression lines obtained with the data.
RESULTS
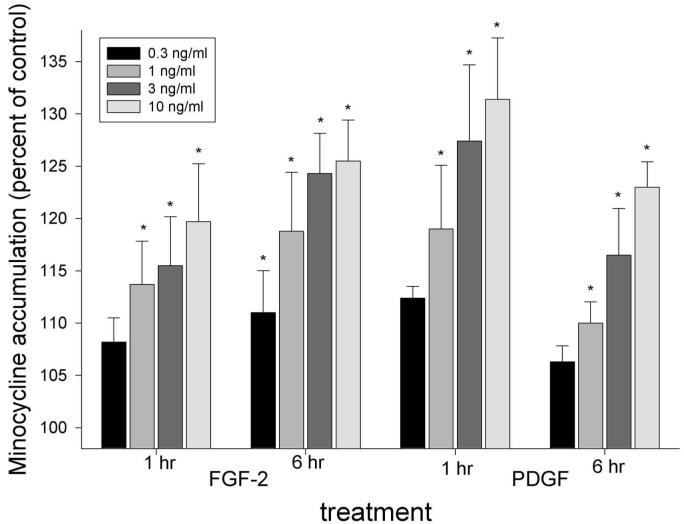
At relatively low concentrations (0.3 to 10 ng/mL), FGF-2 and PDGF induced a significant dose-dependent enhancement of gingival fibroblast minocycline uptake compared with untreated controls (P ≤ 0.003, repeated-measures ANOVA, Fig. 1). FGF-2 (10 ng/mL) enhanced minocycline uptake by 20% after 1 hr of treatment and by 26% after 6 hrs. PDGF (10 ng/mL) produced 31% enhancement after 1 hr and 23% enhancement after 6 hrs.
Figure 1.
Stimulation of fibroblast minocycline accumulation by FGF-2 and PDGF. Confluent fibroblast cultures were starved for 20 hrs and treated with the indicated growth factor concentrations for 1 or 6 hrs. Cell DNA content did not increase significantly under these experimental conditions. After brief incubation at 37°C, 40 μg/mL minocycline was added, and uptake was monitored for 3 min. The data represent the mean ± SEM of 5 individual experiments. Both agents produced a significant treatment effect after 1 and 6 hrs (P < 0.003, repeated-measures ANOVA). Conditions that produced a significant increase in minocycline accumulation compared with controls (P > 0.05, Dunnett’s test) are indicated by *.
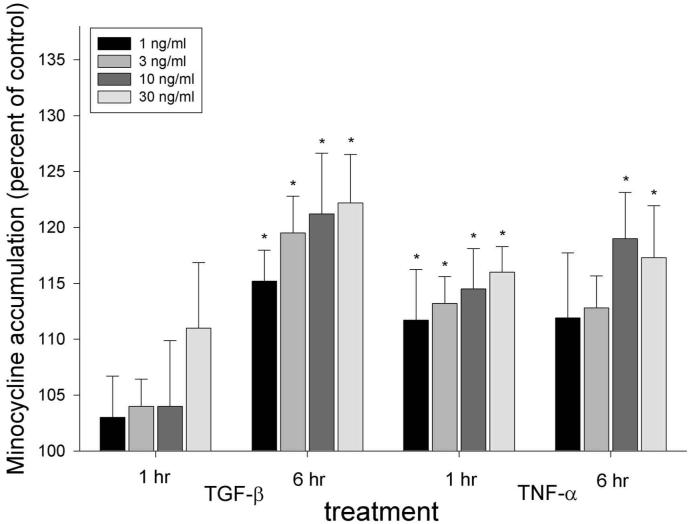
TGF-β (1 to 30 ng/mL, 6 hrs) enhanced minocycline accumulation in a dose-dependent manner (P < 0.001, ANOVA, Fig. 2), but produced no significant effect after 1 hr of treatment (P = 0.24, ANOVA). Treatment for 6 hrs with 10 ng/mL TGF-β enhanced minocycline accumulation by 21%. TNF-α (1 to 30 ng/mL) enhanced minocycline accumulation within 1 hr (P < 0.001, ANOVA), and the effect was sustained through 6 hrs of treatment (P = 0.008). Treatment with 10 ng/mL TNF-α enhanced minocycline accumulation by 15% at 1 hr and by 19% at 6 hrs (Fig. 2).
Figure 2.
Effects of TGF-β1 and TNF-α on fibroblast minocycline accumulation. Experiments were conducted as described in the legend for Fig. 1. The data represent the mean ± SEM of 5 individual experiments. There were no significant effects by TGF at 1 hr (P = 0.24, repeated-measures ANOVA). However, incubation with TGF for 6 hrs produced a significant treatment effect (P < 0.001, repeated-measures ANOVA), and TNF-α produced a significant treatment effect at 1 and 6 hrs (P < 0.001 and P ≤ 0.008, respectively). Conditions that produced a significant increase in minocycline accumulation compared with controls (P > 0.05, Dunnett’s test) are indicated by *.
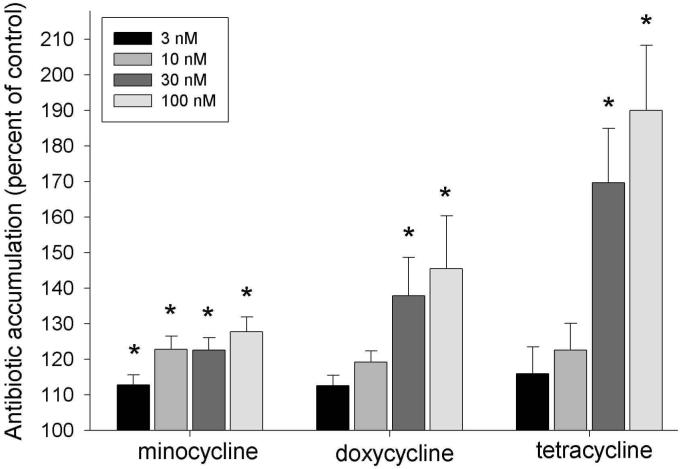
Phorbol esters activate protein kinase C (PKC) in a receptor-independent manner. To determine whether PKC could be involved in signaling for enhanced minocycline uptake, we examined the effects of phorbol myristate acetate (PMA, 3 to 100 nM) on the accumulation of several different tetracyclines by fibroblasts. Brief treatment with PMA significantly enhanced fibroblast uptake of minocycline, doxycycline, and tetracycline (P ≤ 0.002, ANOVA, Fig. 3). Stimulation by 100 nM PMA enhanced minocycline transport by 28%. Under identical conditions, doxycycline transport increased by 45%, and tetracycline transport increased by 90%.
Figure 3.
Enhanced accumulation of tetracyclines by PMA-activated fibroblasts. Confluent fibroblast cultures were starved for 20 hrs and treated with 3 to 100 nM PMA for 15 min prior to assaying transport of minocycline, doxycycline, and tetracycline. The data represent the mean ± SEM of 6 individual experiments. PMA produced a significant treatment effect on the accumulation of all 3 agents (P ≤ 0.002, repeated-measures ANOVA). PMA concentrations indicated by * induced a significant increase in antibiotic accumulation (P > 0.05, Dunnett’s test).
The mechanism of enhancement by growth factors, pro-inflammatory cytokines, and PMA was investigated by kinetic analysis. Untreated control fibroblasts transported minocycline with a Km of 108 μg/mL at a Vmax of 15.2 ng/min/μg cell protein (Table). Treatment with FGF-2, PDGF, TGF-β, TNF-α, and interleukin-1β (IL-1β) all significantly decreased the Km (and increased the affinity) of minocycline transport (P < 0.05, Dunnett’s test). In addition, activation with PMA significantly decreased the Km of transport. None of these factors significantly enhanced the maximum velocity of transport, but TGF-β slowed the velocity of transport significantly (P < 0.05, Dunnett’s test).
Table.
Effects of Biological Mediators and PMA on Kinetic Constantsa for Transport of Minocycline by Gingival Fibroblasts
| Treatment Condition | Kmb (μg/mL) | Vmaxb (ng/min/μg protein) |
|---|---|---|
| Control | 108 ± 4.9 | 15.2 ± 0.5 |
| FGF-2 (10 ng/mL) | 72.3 ± 3.9c | 15.3 ± 1.0 |
| PDGF (10 ng/mL) | 59.5 ± 5.2c | 16.0 ± 0.8 |
| TGFβ (30 ng/mL) | 58.8 ± 6.0c | 11.3 ± 0.8c |
| TNFα (30 ng/mL) | 77.9 ± 5.1c | 12.1 ± 0.5 |
| IL-1β (30 ng/mL) | 75.7 ± 9.8c | 13.5 ± 0.9 |
| PMA (100 nM) | 74.3 ± 6.5c | 18.4 ± 1.2 |
Fibroblasts were pre-treated with the indicated agents, with treatment times as described in the Fig. legends, after which the minocycline transport was assayed in the presence of a range of minocycline concentrations (from 14 to 150 μg/mL). Results were derived from Lineweaver-Burke analysis of transport activity observed during the rapid initial phase of uptake (3 min).
Constants are expressed as mean ± SEM of at least 5 individual experiments. Differences in the means for Km and Vmax were greater than would be expected by chance (P = 0.002, ANOVA).
Within columns, this indicates a value that is significantly different from the control (P < 0.05, Dunnett’s test).
DISCUSSION
Tetracyclines are one of the most widely used classes of antibiotics in periodontics. In addition to their antimicrobial effects, tetracyclines inhibit matrix metalloproteinases (MMPs), which play a role in connective tissue destruction. Through this anti-inflammatory mechanism, low-dose doxycycline therapy enhances the therapeutic response to scaling and root planing (reviewed by Oringer, 2002). The focus of the present work was to determine whether biological mediators associated with wound healing and inflammation could potentially alter the distribution of systemic tetracyclines to the gingiva. Gingival fibroblasts possess a transport system that allows them to take up tetracyclines and act as reservoirs for these agents. The transporter exhibits characteristics similar to those of the widely distributed organic cation transporter family, and can move tetracyclines in the forward or reverse direction to maintain equilibrium between intracellular and extracellular concentrations (Yang et al., 2002). Forward transport into fibroblasts presumably occurs when tetracycline levels are increasing or peaking in the tissue. As antibiotic levels in the blood and tissue decrease from their peak values, the direction of transport reverses in a manner that could maintain relatively high antimicrobial levels in interstitial fluid and GCF (Yang et al., 2002). This could explain why tetracycline, doxycycline, and minocycline levels are higher in GCF than in blood when sampled after blood levels have receded from their peak values (Ciancio et al., 1980; Gordon et al., 1981; Pascale et al., 1986).
Periodontal inflammation, wound healing, and regeneration are coordinated by a multitude of inflammatory mediators and growth factors released by resident cells, platelets, and infiltrating leukocytes. Among the most important are PDGF (a potent fibroblast mitogen), FGF-2 (which stimulates angiogenesis and fibroblast proliferation), TGF-β (which stimulates fibroblast matrix protein synthesis), and TNF-α (which stimulates fibroblast MMP and prostaglandin production) (Birkedal-Hansen, 1993; Cochran and Wozney, 1999). The present study demonstrates that these factors also enhance the uptake of tetracyclines by gingival fibroblasts, effectively increasing the capacity of the tetracycline reservoir in the gingiva. Except for TGF-β, these factors induce a significant increase in fibroblast minocycline accumulation within 1 hr. All 4 significantly enhance minocycline accumulation after 6 hrs of treatment, mainly by inducing an increase in the affinity of fibroblast minocycline transport. This action was not directly related to their effects on cell proliferation, since there was no significant increase in cell DNA over the time course of the experiments.
With its ability to activate PKC directly, phorbol myristate acetate (PMA) is a useful tool for evaluating the role of this signaling pathway in cell regulation. PMA rapidly enhances fibroblast transport of minocycline, doxycycline, and tetracycline, suggesting that PKC is involved in signaling for increased transport activity. The mechanism of enhancement (increased transporter affinity) and the magnitude of the enhancement are similar to those produced by PDGF, FGF-2, TGF-β, and TNF-α. All 4 of these mediators activate a complex cascade of fibroblast signaling pathways, but some of their effects are exerted through activation of PKC (Nanberg et al., 1990; Gorospe et al., 1993; Axmann et al., 1998; Thorsen et al, 2003).
As illustrated in Fig. 3, gingival fibroblasts take up and accumulate several widely used tetracycline antibiotics. Assuming that PDGF, FGF-2, TGF-β, and TNF-α enhance the transport of tetracyclines by gingival fibroblasts in vivo, they could provide a mechanism for preferentially distributing these agents to healing wounds or inflammatory sites. This could benefit the host in several ways. Sustained availability of tetracyclines in wounds and inflammatory sites could enhance the local control of extracellular bacterial infections, which disrupt wound healing and potentiate destructive aspects of the inflammatory response. Local maintenance of tetracycline therapeutic levels in gingiva could also enhance their inhibitory effects on MMPs and reduce extracellular matrix destruction at that site. In addition, cytokine-induced increases in intracellular tetracycline accumulation could potentially enhance the elimination of invasive pathogens associated with aggressive periodontitis. Together, these effects could have a favorable impact on the outcome of periodontal therapy. Although the findings are based on fibroblasts obtained from a single healthy donor and may not be representative of all human gingival fibroblast strains, the present study provides insight into factors that could potentially influence therapeutic levels of tetracyclines in the gingiva. The complex mechanisms that regulate fibroblast tetracycline transport warrant further study in the laboratory.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Angelo Mariotti for providing human gingival fibroblasts. This investigation was supported by USPHS research grant DE12601 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
REFERENCES
- Axmann A, Seidel D, Reimann T, Hempel U, Wenzel KW. Transforming growth factor beta-1-induced activation of the Raf-MEK-MAPK signaling pathway in rat lung fibroblasts via a PKC-dependent mechanisms. Biochem Biophys Res Commun. 1998;249:456–460. doi: 10.1006/bbrc.1998.9188. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993;28:500–510. doi: 10.1111/j.1600-0765.1993.tb02113.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Christersson LA, Albini B, Zambon JJ, Wikesjö UM, Genco RJ. Tissue localization of Actinobacillus actinomycetem-comitans in human periodontitis. 1. Light, immunofluorescence and electron microscopic studies. J Periodontol. 1987;58:529–539. doi: 10.1902/jop.1987.58.8.529. [DOI] [PubMed] [Google Scholar]
- Ciancio SG, Mather ML, McMullen JA. An evaluation of minocycline in patients with periodontal disease. J Periodontol. 1980;51:530–534. doi: 10.1902/jop.1980.51.9.530. [DOI] [PubMed] [Google Scholar]
- Clark RAF. The molecular and cellular biology of wound repair. 2nd ed. Plenum Press; New York: 1996. [Google Scholar]
- Cochran DL, Wozney JM. Biological mediators for periodontal regeneration. Periodontol. 1999;200019:40–58. doi: 10.1111/j.1600-0757.1999.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Fives-Taylor P, Meyer D, Mintz K. Virulence factors of the periodontopathogen Actinobacillus actinomycetemcomitans. J Periodontol. 1996;67:291–297. doi: 10.1902/jop.1996.67.3s.291. [DOI] [PubMed] [Google Scholar]
- Gazzola GC, Dall’Asta V, Franchi-Gazzola R, White MF. The cluster-tray method for rapid measurement of solute fluxes in adherent cultured cells. Anal Biochem. 1981;115:368–374. doi: 10.1016/0003-2697(81)90019-1. [DOI] [PubMed] [Google Scholar]
- Gordon JM, Walker CB, Murphy JC, Goodson JM, Socransky SS. Concentration of tetracycline in human gingival fluid after single doses. J Clin Periodontol. 1981;8:117–121. doi: 10.1111/j.1600-051x.1981.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Gorospe M, Kumar S, Baglioni C. Tumor necrosis factor increases stability of interleukin-1 mRNA by activating protein kinase C. J Biol Chem. 1993;268:6214–6220. [PubMed] [Google Scholar]
- Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal destruction. J Periodontol. 2003;74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- Lever M. Improved fluorometric determination of tetracyclines. Biochem Med. 1972;6:216–222. doi: 10.1016/0006-2944(72)90041-5. [DOI] [PubMed] [Google Scholar]
- Mariotti A, Cochran DL. Characterization of fibroblasts derived from human periodontal ligament and gingiva. J Periodontol. 1990;61:103–111. doi: 10.1902/jop.1990.61.2.103. [DOI] [PubMed] [Google Scholar]
- Nanberg E, Morris C, Higgins T, Vara F, Rozengurt E. Fibroblast growth factor stimulates protein kinase C in quiescent 3T3 cells without Ca2+ mobilization or inositol phosphate accumulation. J Cell Physiol. 1990;143:232–242. doi: 10.1002/jcp.1041430206. [DOI] [PubMed] [Google Scholar]
- Oringer RJ. Modulation of the host response in periodontal therapy. J Periodontol. 2002;73:460–470. doi: 10.1902/jop.2002.73.4.460. [DOI] [PubMed] [Google Scholar]
- Pascale D, Gordon J, Lamster I, Mann P, Seiger M, Arndt W. Concentration of doxycycline in human gingival fluid. J Clin Periodontol. 1986;13:841–844. doi: 10.1111/j.1600-051x.1986.tb02240.x. [DOI] [PubMed] [Google Scholar]
- Pavicic MJ, van Winkelhoff AJ, deGraaff J. In vitro susceptibilities of Actinobacillus actinomycetemcomitans to a number of antimicrobial combinations. Antimicrob Agents Chemother. 1992;36:2634–2638. doi: 10.1128/aac.36.12.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen VA, Vorland M, Bjorndal B, Bruland O, Holmsen H, Lillehaug J. Participation of phospholipase D and alpha/beta-protein kinase C in growth factor-induced signaling in C3H10T1/2 fibroblasts. Biochim Biophys Acta. 2003;1632:62–71. doi: 10.1016/s1388-1981(03)00063-5. [DOI] [PubMed] [Google Scholar]
- Van Winkelhoff AJ, Rams TE, Slots J. Systemic antibiotic therapy in periodontics. Periodontol 2000. 1996;10:45–78. doi: 10.1111/j.1600-0757.1996.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Yang Q, Nakkula RJ, Walters JD. Accumulation of ciprofloxacin and minocycline by cultured human gingival fibroblasts. J Dent Res. 2002;81:836–840. doi: 10.1177/154405910208101208. [DOI] [PMC free article] [PubMed] [Google Scholar]