Abstract
Background and purpose:
Many drugs associated with acquired long QT syndrome (LQTS) directly block human ether-a-go-go-related gene (hERG) K+ channels. Recently, disrupted trafficking of the hERG channel protein was proposed as a new mechanism underlying LQTS, but whether this defect coexists with the hERG current block remains unclear. This study investigated how ketoconazole, a direct hERG current inhibitor, affects the trafficking of hERG channel protein.
Experimental approach:
Wild-type hERG and SCN5A/hNav 1.5 Na+ channels or the Y652A and F656C mutated forms of the hERG were stably expressed in HEK293 cells. The K+ and Na+ currents were recorded in these cells by using the whole-cell patch-clamp technique (23°C). Protein trafficking of the hERG was evaluated by Western blot analysis and flow cytometry.
Key results:
Ketoconazole directly blocked the hERG channel current and reduced the amount of hERG channel protein trafficked to the cell surface in a concentration-dependent manner. Current density of the hERG channels but not of the hNav 1.5 channels was reduced after 48 h of incubation with ketoconazole, with preservation of the acute direct effect on hERG current. Mutations in drug-binding sites (F656C or Y652A) of the hERG channel significantly attenuated the hERG current blockade by ketoconazole, but did not affect the disruption of trafficking.
Conclusions and implications:
Our findings indicate that ketoconazole might cause acquired LQTS via a direct inhibition of current through the hERG channel and by disrupting hERG protein trafficking within therapeutic concentrations. These findings should be considered when evaluating new drugs.
Keywords: hERG channel, hERG protein trafficking, ketoconazole, acquired long QT syndrome, K+ channels
Introduction
In human cardiac ventricular cells, the principal repolarizing currents activated during the action potential plateau are the rapidly (IKr) and slowly (IKs) activating components of the delayed rectifier K+ current (Sanguinetti and Jurkiewicz, 1990; Carmeliet, 1993). IKr is encoded by the human ether-a-go-go-related gene (hERG or KCNH2; Curran et al., 1995; Sanguinetti et al., 1995; Trudeau et al., 1995). In addition to class III antiarrhythmic drugs, a remarkable array of structurally diverse therapeutic agents that induce acquired long QT syndrome (LQTS) block hERG or IKr channels via common drug-binding sites (Y652 or F656) within the pore-S6 helices of the hERG channel (Mitcheson et al., 2000). Pentamidine and arsenic trioxide reportedly disrupt hERG protein trafficking to the cell surface membrane, despite having a limited hERG channel-blocking activity (Ficker et al., 2004; Cordes et al., 2005; Kuryshev et al., 2005). Moreover, celastrol and fluoxetin block hERG current and disrupt hERG protein trafficking (Rajamani et al., 2006; Sun et al., 2006).
Ketoconazole is a widely prescribed azole antifungal drug available in topical, vaginal and parenteral formulae. Azole antifungals (for example, fluconazole, ketoconazole and miconazole) are associated with acquired LQTS and ventricular arrhythmias (Viskin, 1999; Roden, 2001; Yap and Camm, 2003). These agents inhibit multiple cytochrome P450 enzymes in the liver and gastrointestinal tract (Dresser et al., 2000; Venkatakrishnan et al., 2000; Zhang et al., 2002), thus their mechanism of arrhythmogenesis is likely to involve a rise in the plasma concentrations of QT interval-prolonging drugs (such as class III antiarrhythmic drugs, some H1-receptor antagonists and antibiotics) that use the same metabolic pathway (Zimmermann et al., 1992; Honig et al., 1993; Tsai et al., 1997; Albengres et al., 1998; Tonini et al., 1999). However, ventricular arrhythmia may occur without concomitant risk factors or other QT interval-prolonging drugs (Mok et al., 2005), suggesting that azole antifungal drugs affect the heart directly. In fact, ketoconazole directly blocks hERG channels (Dumaine et al., 1998; Ekins et al., 2002; Yao et al., 2005; Ridley et al., 2006), although it remains unclear whether ketoconazole also disrupts hERG protein trafficking to the cell surface.
The present study investigated the possible coexistence of a hERG current block and protein trafficking defect in drug-induced acquired LQTS. The results showed that ketoconazole inhibits the hERG current via common drug-binding sites, and also disrupts channel protein trafficking to reduce the surface membrane expression of hERG channels. Common drug-binding sites are not involved in the trafficking defect.
Methods
hERG and SCN5A channel expression
Wild-type hERG and SCN5A (human cardiac voltage-gated Na+ channel gene) encoded hNav 1.5 Na+ channels were stably expressed in a human embryonic kidney (HEK293) cell line, as described previously (Nagatomo et al., 1998; Zhou et al., 1998). Y652A (tyrosine to alanine substitution at position 652) and F656C (phenylalanine to cysteine substitution at position 656) mutations were generated by site-directed mutagenesis of wild-type hERG cDNA, as described previously (Kikuchi et al., 2005). HEK293 cells were transfected with these constructs using Lipofectamine (Invitrogen, San Diego, CA, USA). Stably transfected cells generated through G418 (Calbiochem, Darmstadt, Germany) antibiotic selection were subcloned to achieve a uniform expression level. The cells were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. For electrophysiological analyses, cells were harvested from the culture dish by trypsinization, washed with Dulbecco's modified Eagle's medium and stored in this medium at room temperature for use within 8 h of harvest.
Electrophysiological recordings
The hERG K+ and hNav 1.5 Na+ currents were recorded using the whole-cell patch-clamp technique at room temperature (23±1 °C). Transfected cells were transferred to a bath mounted on the stage of an inverted microscope (Diaphot; Nikon, Tokyo, Japan). For hERG current recordings, the bath was perfused with HEPES-buffered Tyrode solution containing (in mM) NaCl 137, KCl 4, CaCl2 1.8, MgCl2 1, glucose 10, and HEPES 10 (pH 7.4). The internal pipette solution comprised (in mM) KCl 130, MgCl2 1, EGTA 5, Mg-ATP 5 and HEPES 10 (pH 7.2). The electrodes were constructed from borosilicate glass using a micropipette puller (P-87; Sutter Instrument Co., Novato, CA, USA) and heat-polished with a microforge (MF-83; Narishige, Tokyo, Japan). The final resistance of the electrode was 3–5 MΩ when filled with the pipette solution. For Na+ current recordings, the bath solution contained (in mM) NaCl 140, KCl 4, CaCl2 1.8, MgCl2 0.75 and HEPES 5 (pH 7.4), and the pipette solution contained (in mM) CsF 120, CsCl 20, EGTA 5 and HEPES 5 (pH 7.4). The resistance of the electrode was 1–1.5 MΩ when filled with the pipette solution. The methods used to achieve and verify voltage control were as published previously (Nagatomo et al., 1998). The volume of the bath was 0.6 ml and the external solution in the bath was almost completely exchanged within 1 min at a perfusion rate of 1.25 ml min−1. Membrane currents were recorded with an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA) and digitized at 2–100 kHz with an analogue-to-digital converter (DigiData 1200B; Axon Instruments). A computer software (pCLAMP Ver 8.1; Axon Instruments) was used to generate voltage clamp protocols, acquire data and analyze current traces.
Western blot analysis
Ketoconazole was diluted in serum- and antibiotic-free culture medium and added to hERG-HEK cells for 48 h (with a media change after 24 h) at 37 °C before analysis by Western blotting. The cells were washed in phosphate-buffered saline, then lysed in buffer (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% deoxycholate, 1% Triton-X-100, 2 mM EDTA, 0.1% SDS and 50 mM NaF). Protein concentrations were determined by the bicinchoninic acid method (Pierce Chemicals, Rockford, IL, USA). Protein (10 μg) per sample was electrophoresed on 3–8% Tris-acetate gels (NuPAGE; Invitrogen) and transferred onto nitrocellulose membranes (Invitrogen). The membranes were blocked with 10% non-fat dry milk in Tris-buffered saline containing 0.2% Tween 20 (TBS-T) overnight, before incubation with rabbit anti-hERG antibody (1:1000, SC-20136; Santa Cruz Biotechnology, Santa Cruz, CA, USA), recognizing the hERG channel protein N-terminus, in 5% non-fat dry milk/TBS-T for 1 h at room temperature. The membranes were then washed with TBS-T and incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:5000, SC-2004; Santa Cruz Biotechnology) in 5% non-fat dry milk/TBS-T for 1 h at room temperature. After washing with TBS-T, the membranes were developed using Super Signal West Pico chemiluminescent substrate (Pierce Chemicals). To normalize the hERG expression levels to a reference protein, the membranes were also probed with a rabbit anti-β-actin antibody (1:1000, no. 4976; Cell Signalling Technology, Danvers, MA, USA). Blots were analyzed and quantified using Scion Image System (Scion Corporation, Frederick, MD, USA).
Flow cytometry and quantification of cell surface expression
The cells were harvested using trypsin as described earlier, washed in medium (phosphate-buffered saline+0.5% albumin) and incubated with rabbit anti-hERG-extracellular antibody (1:100, APC-109; Alomone Labs, Jerusalem, Israel) on ice for 30 min. The cells were washed twice in medium and incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibody (1:100, A11008; Molecular Probes, Eugene, OR, USA) on ice for 30 min. To subtract nonspecific binding, control and ketoconazole-treated cells were also incubated with fluorescein isothiocyanate-labelled mouse anti-IgG antibody (BD Biosciences, San Jose, CA, USA) on ice for 30 min. Finally, the cells were washed twice in medium, and surface fluorescence was measured on an EPICS XL flow cytometer and analyzed using SYSTEM II SOFTWARE (Beckman Coulter, Fullerton, CA, USA). The value of mean fluorescence intensity obtained from anti-IgG-labelled cells was subtracted as a background from that obtained from each test sample (first and secondary antibody-labelled cells) at each concentration.
Statistical analysis
Data are expressed as mean±s.e.m. Where applicable, n represents the number of cells studied. Curve fitting was performed using multiple nonlinear least-squares regression analysis (pCLAMP ver. 8.1, Axon Instruments or Sigma Plot ver 7.0, SPSS Science, Chicago, IL, USA). Statistical significance was analyzed using the two-tailed Student's t-test (paired or unpaired) for comparisons of two means and ANOVA for comparison of multiple means. P-values <0.05 were considered statistically significant.
Chemicals
Ketoconazole was (purchased from Sigma-Aldrich) dissolved in dimethyl sulphoxide to prepare a stock solution (100 mM). Final drug concentrations were prepared by diluting stock solutions with Tyrode solution. The highest concentration of dimethyl sulphoxide used in this study was 0.1%. In preliminary experiments, hERG tail current amplitudes expressed in HEK293 cells were not significantly changed after 3 min of dimethyl sulphoxide application at 0.1% (n=4).
Results
Ketoconazole directly inhibits hERG channels via Y652 and F656 residues
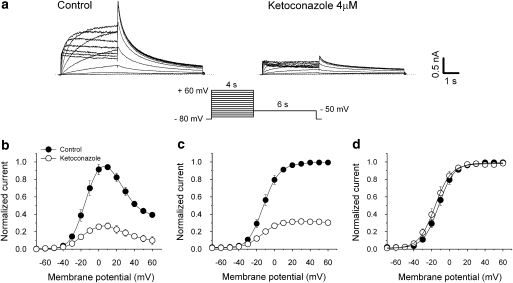
The effect of ketoconazole on the hERG current–voltage (I–V) relationship is shown in Figure 1. The hERG current was elicited from a holding potential of −80 mV by 4-s-long depolarizing steps between −70 and 60 mV applied in 10-mV increments every 15 s. Tail current was recorded with a step to −50 mV for 6 s. Representative currents recorded under control conditions and 3 min after application of ketoconazole (4 μM) in the same cell are shown in Figure 1a. Ketoconazole reduced hERG current amplitude and tail current during the depolarizing steps (Figures 1b and c). Control hERG current during the depolarizing steps reached a peak level at 0–10 mV, with tail current fully activated following steps to 10–20 mV. Ketoconazole (4 μM) reduced the steady-state current amplitude to 28.1±2.8% (n=6) of control at 10 mV, and tail current peak amplitude to 32.5±2.2% (n=6) of control at 20 mV. We also evaluated the voltage dependence of activation by plotting normalized tail current as a function of voltage (Figure 1d). Data were fitted with Boltzmann function: I=Imax[1+exp(V1/2−V)/κ]−1, where Imax, V1/2 and κ are maximum amplitude, half-activation voltage and slope factor, respectively. In this protocol, ketoconazole neither changed the half-activation voltage nor the slope factor.
Figure 1.
Current–voltage relationship for hERG channels and ketoconazole block. (a) hERG currents under control conditions and in the presence of 4 μM ketoconazole were recorded using the pulse protocol shown. (b, c) Normalized (to control values) I–V relationships for current measured at the end of depolarizing steps (b) and tail currents (c) in control and 4 μM ketoconazole-treated cells (n=6). (d) Peak tail currents were normalized to their respective maximum current amplitude (control and drugs) to illustrate changes in half-maximal activation voltages. Solid lines represent fits to Boltzmann function. Ketoconazole neither changed the half-activation voltage nor the slope factor (V1/2: –11.6±2.2 and –14.7±2.5 mV; κ. 8.2±0.4 and 8.4±0.5; n=6, in controls and after ketoconazole, respectively; P>0.05). Data are mean±s.e.m.
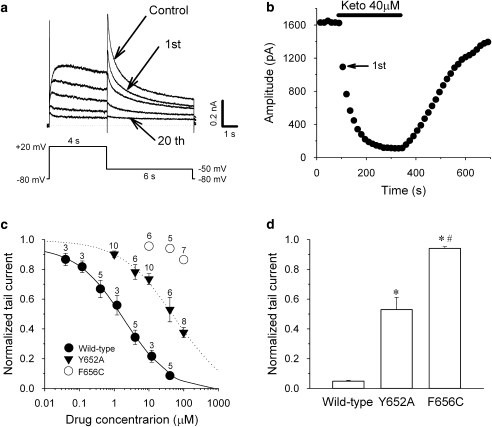
Figure 2a shows the effects of ketoconazole (40 μM) on the hERG current during a drug wash-in protocol. The hERG current was elicited from a holding potential of −80 mV by a 4-s depolarizing step to 20 mV followed by a repolarizing step to −50 mV for 6 s, and the protocol was applied every 15 s. After establishing control recordings, ketoconazole was applied to the bath. The hERG channel block occurred rapidly to reach a steady-state level within 2–3 min (Figure 2b), and was reversed promptly upon drug washout.
Figure 2.
Ketoconazole inhibits hERG current by accessing the Y652 and F656 amino-acid residues. (a) Representative current traces under control conditions and after ketoconazole treatment (40 μM; 1st, 2nd, 3rd, 4th and 20th current traces are shown). (b) Time course of ketoconazole-induced hERG peak tail current inhibition from the same cell as in panel a. (c) Concentration–response relationships for peak tail current in hERG wild-type channels, as well as in Y652A and F656C mutants. Currents in the presence of ketoconazole were normalized to the control amplitudes and plotted as a function of drug concentration. Lines represent fits with the Hill equation. Data are mean±s.e.m. The numbers by each symbol represent the number of cells tested. (d) Comparison of hERG current inhibition by 40 μM ketoconazole with wild-type (n=5), Y652A (n=6) and F656C (n=5) mutant channels. The hERG currents evaluated at peak tail current amplitude in the presence of ketoconazole were normalized to respective control values. Data are mean±s.e.m. *P<0.05, compared with the wild-type. #P<0.05, Y652A vs F656C.
In the next step, we recorded changes in hERG peak tail current amplitude at 5 min after application of the drug to assess the concentration–response relationship of the ketoconazole block. Concentration–response curves were obtained by using the Hill equation Idrug/Icontrol=1/[1+(D/IC50)n], where D is the drug concentration, IC50 is the drug concentration required to attain a 50% block and n is the Hill coefficient. Ketoconazole reduced hERG current in a concentration-dependent manner (Figure 2c). The IC50 value and Hill coefficient for tail current peak amplitude were 1.92 μM and 0.63, respectively.
We also assessed the molecular basis of ketoconazole binding to hERG channels using the hERG Y652A and F656C mutant channels. The concentration–response relationships for the mutated channels were plotted simultaneously with those of wild-type channels (Figure 2c). The IC50 value and Hill coefficient for Y652A mutant channels were 56.2 μM and 0.63, respectively. The high concentrations of drug required to inhibit the F656C channels precluded obtaining IC50 values in these cells. Therefore, the affinities of ketoconazole for hERG wild-type and mutant channels were compared using ketoconazole at 40 μM (Figure 2d). The mean relative tail current amplitudes after application of ketoconazole were significantly different in wild-type, Y652A and F656C channels. Thus, the inhibitory effect of ketoconazole on hERG current was almost completely abolished in the F656C mutant channels and partially attenuated in the Y652A mutant channels, indicating that ketoconazole inhibits hERG current via putative common sites for drug binding.
Disruption of hERG protein trafficking
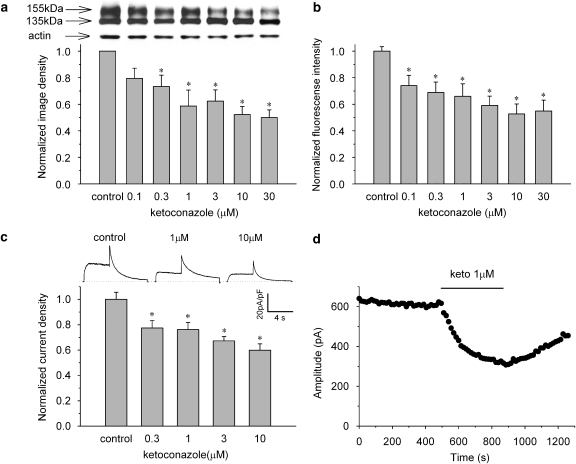
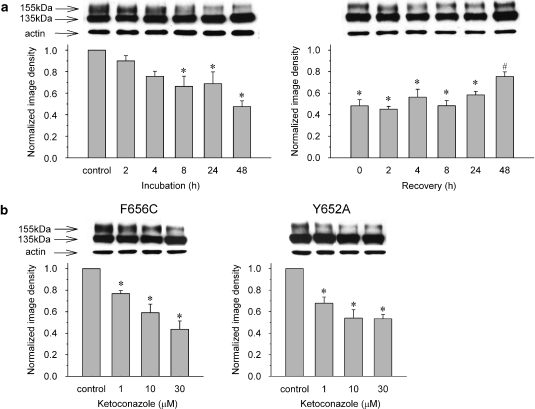
We next evaluated the effects of ketoconazole on hERG channel protein trafficking. The cells were incubated in control or ketoconazole-containing media (0.1–30 μM) for 48 h and then subjected to western blot analysis. Figure 3a (upper panel) shows representative cell lysates probed for hERG. Wild-type channels showed protein bands at ∼135 kDa (immature, core-glycosylated channel protein) and at ∼155 kDa (mature, complexly glycosylated channel protein). The intensity of the 155-kDa band decreased with increased ketoconazole concentrations, whereas the 135-kDa band remained unchanged. The protein bands were quantified by densitometry, and after correcting for the corresponding actin levels, were normalized to control, drug-free conditions. The normalized image density of mature hERG protein was significantly decreased in a concentration-dependent manner (n=6, P<0.05 by ANOVA; Figure 3a).
Figure 3.
Ketoconazole reduced maturation and surface expression of hERG channels. Concentration-dependent decrease of 155-kDa hERG protein (a), fluorescence intensity of antibody-labelled hERG channels detected by flow cytometry (b) and current densities (c). The cells were incubated for 48 h in control or ketoconazole-containing medium. Panel a, upper panel: representative western blot analysis. Data are mean±s.e.m. *P<0.05, compared with the control (n=6). Panel b, data are mean±s.e.m. *P<0.05, compared with the control (n=9). Panel c, upper lane: representative current traces under control conditions and after long-term application of ketoconazole at 1 and 10 μM (see Figure 2a for pulse protocol). The hERG current density was measured after 1 h following the washing of the drug. Peak tail current densities at each concentration were normalized to the value in control conditions. Data are mean±s.e.m. *P<0.05, compared with the control (n=16–21). (d) Time course of direct hERG current block by 1 μM ketoconazole after 48-h incubation with 1 μM ketoconazole. Amplitude of the peak tail current was plotted against time (see Figure 2a for pulse protocol). An acute hERG current block occurred after disruption of hERG protein trafficking.
The cell surface expression of the hERG protein was then quantified by flow cytometry with an anti-hERG antibody that recognizes the extracellular portion of the hERG channels. The fluorescence intensities at each concentration were normalized to the value in control conditions. The normalized fluorescence intensities of antibody-labelled hERG channels in the cell membrane decreased in a concentration-dependent manner (n=9, P<0.05 by ANOVA; Figure 3b). The results indicate that ketoconazole inhibited the maturation or trafficking of hERG channels, resulting in reduced cell surface expression.
To validate the biochemical results, we studied the functional effects of ketoconazole on hERG current. The cells were incubated in control or ketoconazole-containing medium (0.3–10 μM) for 48 h and hERG current density was measured after 1 h following the washing of the drug. Figure 3c (top) shows representative current traces elicited by the same voltage protocol described in Figure 2a. The peak tail current densities at each concentration normalized to the value under control conditions were also decreased in a concentration-dependent manner (n=16–21 at each concentration, P<0.05 by ANOVA; Figure 3c). These results indicate that the number of functional channels on the cell surface correlated well with the defect in hERG channel trafficking. Figure 3d shows the time course of direct hERG current blockage by 1 μM ketoconazole after 48-h incubation with 1 μM ketoconazole. The acute block was additive even after a decrease in hERG protein expression on the surface membrane, and the current inhibition was reversed promptly upon drug washout. Thus, the defective trafficking induced by long-term application of ketoconazole was independent from the acute hERG current block.
Acute and chronic effects of ketoconazole on the Na+ channels
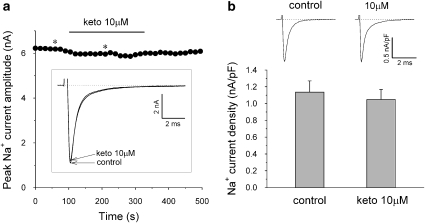
To investigate whether the effects of ketoconazole were specific to hERG channels, we tested hNav 1.5 Na+ channel currents. Figure 4a shows the acute direct effects of ketoconazole (10 μM) on the Na+ current during a drug wash-in protocol. Na+ current was elicited by a 24-ms depolarization step to −20 mV from a holding potential of −150 mV, with the protocol applied every 15 s. Application of 10 μM ketoconazole did not change significantly the amplitude of the peak inward Na+ current. We next tested the long-term effect of ketoconazole on the Na+ current density (Figure 4b). The cells were incubated in control or ketoconazole-containing medium (10 μM) for 48 h and Na+ current density was measured after 1 h following the washing of the drug. At 10 μM, ketoconazole did not significantly alter the current density of the peak Na+ current after incubation of cells for 48 h with ketoconazole.
Figure 4.
Acute and chronic effects of ketoconazole on the hNav 1.5 Na+ channel. (a) Time course of peak inward Na+ current amplitude during ketoconazole (10 μM) wash-in protocol. Representative current recordings indicated by asterisks are shown in the box. (b) Upper lane: representative Na+ current traces under control conditions and after cell incubation with 10 μM ketoconazole for 48 h. The Na+ current density was measured after 1 h following the washing of the drug. The Na+ current was elicited by a 24-ms depolarization step to –20 mV from a holding potential of –150 mV. Ketoconazole treatment did not significantly alter the current density of the peak Na+ current. Data are mean±s.e.m. (n=13 in controls and n=16 after ketoconazole).
Time course of disruption and recovery of hERG protein trafficking
We investigated the biogenesis of the ketoconazole-induced alteration in hERG channel trafficking by Western blot analysis. The mature hERG protein band showed a time-dependent decrease in 30 μM ketoconazole and recovery following drug washout from the culture medium (Figure 5a). Densitometry of the blots expressed relative to the control showed a reduction in mature protein of 69±10.9 and 48±5.3% after 24 and 48 h of incubation with ketoconazole, respectively. Following drug washout, the relative image densities of mature hERG protein increased to 62±12.9 and 76±7.4% of control by 24 and 48 h, respectively.
Figure 5.
(a) Western blotting analysis of the development (left) and recovery (right) of 30 μM ketoconazole-induced disruption of hERG protein trafficking over time. Data are mean±s.e.m. *P<0.05, compared with the control, #P<0.05, compared with just after washing of the drug (recovery 0), 2, 4, 8 and 24 h (n=4–6). (b) Western blotting of cells expressing hERG F656C and Y652A mutant channels under control conditions, and following 48 h incubation with 1–30 μM ketoconazole. Data are mean±s.e.m. *P<0.05, compared with the control (n=6).
Finally, we tested whether putative drug-binding site mutation, which was confirmed by the electrophysiological study, altered ketoconazole-induced disruption of protein trafficking. Cells expressing the F656C and Y652A mutant channel proteins were incubated in 1–30 μM ketoconazole for 48 h and compared to control (untreated) cells by western blotting (Figure 5b). As observed earlier with the wild-type hERG protein (see Figure 3a), the normalized image density of the 155-kDa band was significantly decreased in a concentration-dependent manner in both F656C and Y652A mutant channels (n=6, P<0.05 by ANOVA; Figure 5b). The results indicate that these common drug-binding sites in the hERG protein are not involved in the altered trafficking induced by ketoconazole.
Discussion
The present study demonstrated that the azole antifungal drug ketoconazole directly inhibits both the cellular hERG K+ current and hERG channel protein trafficking at similar drug concentrations. Common drug-binding sites (Y652 and F656) are involved in the direct hERG channel block, but not in the hERG trafficking defect.
Ketoconazole has been associated with acquired LQTS and reported to directly inhibit hERG channels expressed in Xenopus oocytes and in mammalian cells (Dumaine et al., 1998; Ekins et al., 2002; Yao et al., 2005; Ridley et al., 2006). In the present experiments, ketoconazole also directly inhibited hERG current in HEK cells with an IC50 of 1.92 μM. The IC50 value was in good agreement with that (1.7–2.0 μM) reported previously using HEK293 cells (Ekins et al., 2002; Yao et al., 2005; Ridley et al., 2006). The gating mechanisms of hERG channel blockage by ketoconazole were similar to those reported by Ridley et al. (2006), in that a decelerated slow component of deactivation, accelerated activation kinetics and open state dependence were observed (data not shown). Moreover, the specific amino-acid residues (F656 and Y652) were implicated in acute direct hERG current inhibition by ketoconazole. However, ketoconazole inhibited Y652A channels with an IC50 value of 56.2 μM and did not significantly inhibit the F656C channels in the present study, while Ridley et al. (2006) reported that it inhibited Y652A and F656A channels with IC50 values of 7.3 and 51.5 μM, respectively. We measured current amplitude at room temperature but Ridley et al. measured it at 37 °C. Although it has been reported that temperature differences do not affect the affinity of ketoconazole in the wild-type channels (Yao et al., 2005) and in fact the IC50 values were not significantly different between studies (1.92 vs 1.7 μM), the difference in temperature values used in the two sets of experiments might explain the difference in the affinity of ketoconazole to the Y652 and F656 mutated channels. Another possible reason for the difference in IC50 of the two studies is the difference in pulse protocol and mutant channel used. Ridley et al. (2006) used a strong negative pulse protocol (−120 mV) with high extracellular K+ solution (94 mM) to measure inward tail current because of the low expression of the F656A channels, while we followed the same protocol used in the wild-type channels because the expression of F656C channels was not significantly different from wild-type channels, in our cells. Since the potency of hERG channel blockers varies depending on the experimental temperature and the pulse protocol (Yao et al., 2005), complex factors might affect drug affinity in the mutated channels.
Structurally and therapeutically diverse agents have been shown to disrupt post-translational hERG channel protein processing, thereby reducing channel density at the cell surface (Ficker et al., 2004; Cordes et al., 2005; Kuryshev et al., 2005; Rajamani et al., 2006; Sun et al., 2006). Arsenic trioxide and pentamidine inhibit hERG protein trafficking rather than directly inhibiting the channel action (Ficker et al., 2004; Cordes et al., 2005; Kuryshev et al., 2005). On the other hand, celastrol and fluoxetine inhibit both ion conductivity through the hERG channels and protein trafficking, although the latter effect was ∼5- to 10-fold more potent than the current-blocking effect in celastrol (Rajamani et al., 2006; Sun et al., 2006). In the present study, both the acute and chronic effects were observed at concentrations more than 0.1 μM and the sensitivity of ketoconazole for the disruption of protein trafficking was similar to that for the direct hERG channel block. As the peak plasma concentrations range from 1.5 to 3.1 μg/ml (3.1–5.8 μM) after a single 200 mg dose of ketoconazole (Como and Dismukes, 1994), clinically relevant concentrations of ketoconazole could disrupt hERG protein trafficking.
Mok et al. (2005) reported a case of ketoconazole-induced torsades de pointes without concomitant use of other QT-prolonging drugs. The patient showed a marked corrected QT interval prolongation (580 ms) 5 days after taking ketoconazole at a dose of 200 mg daily, and normalization of the QT interval occurred more than 4 days after withdrawal of ketoconazole. Since the terminal elimination half-life for ketoconazole (dose of 200 mg daily) at steady-state conditions is 7–10 h (Como and Dismukes, 1994), it is difficult to attribute this case solely to acute direct hERG channel blockage and long-term effects of ketoconazole might be involved. Our finding that ketoconazole disrupts the cell surface expression of hERG channels thus provides a plausible explanation for this case of ketoconazole-induced acquired LQT. Our data also provide a hint as to the slow recovery of normal QT interval in this case, since the mature hERG protein in our treated cells was not restored completely even after 48 h of drug washout.
The hERG channel proteins are synthesized in the endoplasmic reticulum and transported to the cell surface via the Golgi apparatus. Misfolded and incompletely assembled proteins are retained and degraded in the endoplasmic reticulum. The pharmacological effect of ketoconazole on the cell surface expression of hERG may therefore reflect a defect in protein synthesis and/or trafficking or an increased turnover of the membrane protein. The unchanged state of the immature form of hERG (135-kDa band by western blot) would argue against an effect on channel synthesis. It is not clear from these findings how ketoconazole interferes with hERG trafficking and maturation, and further studies will be necessary to elucidate the underlying biochemical mechanisms.
The structural requirement for drug binding in hERG channels was characterized in detail recently (Lees-Miller et al., 2000; Mitcheson et al., 2000; Kamiya et al., 2001; Sanchez-Chapula et al., 2002). Aromatic amino-acid residues (Y652 and particularly F656) in the S6 domain are the most important molecular determinants of drug binding (Mitcheson et al., 2000). In the present study, F656C mutant channels markedly attenuated hERG current inhibition by ketoconazole. The result clearly showed that the binding domain in the pore-S6 region of hERG mediates the direct ketoconazole-induced current block. However, the ketoconazole-induced trafficking defect was unaffected by the F656C mutation, suggesting a different interaction site on the hERG protein or a different protein in the secretory pathway regulating hERG processing.
The present study had certain limitations. First, we expressed the hERG and the hNav 1.5 proteins heterologously in a human cell line because these channels are common targets for the drug-induced acquired QT syndrome. The possible effects of ketoconazole on other ion channels as well as on receptors in vivo cannot be excluded, and thus studies on endogenous hERG channel in cardiomyocytes, especially the effects of ketoconazole on the duration of action potential, would be desirable. Second, the reported plasma protein binding for ketoconazole is approximately 99% (Como and Dismukes, 1994). Our concentration–response data thus suggest that serum drug levels would have minimal effects on hERG current and trafficking under normal conditions. Thus, the apparent low incidence of QT prolongation by ketoconazole might in part be accounted for by its high level of binding to plasma protein. Nonetheless, our findings provide an additional cellular mechanism for ketoconazole-induced long QT interval prolongation and cardiac arrhythmias. Inhibition of the hERG channels may therefore be of greater concern when ketoconazole is used in patients with congenital LQTS, in patients with electrolyte abnormalities such as hypokalaemia or in patients treated with other QT interval-prolonging drugs, regardless of their mechanism of action.
In summary, therapeutic ketoconazole might cause acquired LQTS by direct blocking of the hERG channel current and by disrupting hERG protein trafficking. Drug safety testing for evaluating new drugs should thus employ screening protocols that detect acute and chronic effects, reflecting the different mechanisms of action.
Acknowledgments
We thank Drs Henry J Duff, Zhengfeng Zhou and Qiuming Gong for the expert technical assistance and advice. This work was supported by Astrazeneca Research Grant (to TN) and by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to TN 17590194).
Abbreviations
- HEK
human embryonic kidney
- hERG
human ether-a-go-go-related gene
- LQTS
long QT syndrome
- TBS
Tris-buffered saline
Conflict of interest
The authors declare no conflict of interest.
References
- Albengres E, Le Louet H, Tillement JP. Systemic antifungal agents. Drug interactions of clinical significance. Drug Saf. 1998;18:83–97. doi: 10.2165/00002018-199818020-00001. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Mechanisms and control of repolarization. Eur Heart J. 1993;14 Suppl H:3–13. doi: 10.1093/eurheartj/14.suppl_h.3. [DOI] [PubMed] [Google Scholar]
- Como JA, Dismukes WE. Oral azole drugs as systemic antifungal therapy. N Engl J Med. 1994;330:263–272. doi: 10.1056/NEJM199401273300407. [DOI] [PubMed] [Google Scholar]
- Cordes JS, Sun Z, Lloyd DB, Bradley JA, Opsahl AC, Tengowski MW, et al. Pentamidine reduces hERG expression to prolong the QT interval. Br J Pharmacol. 2005;145:15–23. doi: 10.1038/sj.bjp.0706140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- Dresser GK, Spence JD, Bailey DG. Pharmacokinetic–pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38:41–57. doi: 10.2165/00003088-200038010-00003. [DOI] [PubMed] [Google Scholar]
- Dumaine R, Roy ML, Brown AM. Blockade of HERG and Kv1.5 by ketoconazole. J Pharmacol Exp Ther. 1998;286:727–735. [PubMed] [Google Scholar]
- Ekins S, Crumb WJ, Sarazan RD, Wikel JH, Wrighton SA. Three-dimensional quantitative structure–activity relationship for inhibition of human ether-a-go-go-related gene potassium channel. J Pharmacol Exp Ther. 2002;301:427–434. doi: 10.1124/jpet.301.2.427. [DOI] [PubMed] [Google Scholar]
- Ficker E, Kuryshev YA, Dennis AT, Obejero-Paz C, Wang L, Hawryluk P, et al. Mechanism of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol. 2004;66:33–44. doi: 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- Honig PK, Wortham DC, Zamani K, Conner DP, Mullin JC, Cantilena LR. Terfendine–ketoconazole interaction. Pharmacokinetic and electrocardiographic consequences. JAMA. 1993;269:1513–1518. [PubMed] [Google Scholar]
- Kamiya K, Mitcheson JS, Yasui K, Kodama I, Sanguinetti MC. Open channel block of HERG K+ channels by vesnarinone. Mol Pharmacol. 2001;60:244–253. doi: 10.1124/mol.60.2.244. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Nagatomo T, Abe H, Kawakami K, Duff HJ, Makielski JC, et al. Blockade of HERG cardiac K+ current by antifungal drug miconazole. Br J Pharmacol. 2005;144:840–848. doi: 10.1038/sj.bjp.0706095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryshev YA, Ficker E, Wang L, Hawryluk P, Dennis AT, Wible BA, et al. Pentamidine-induced long QT syndrome and block of HERG trafficking. J Pharmacol Exp Ther. 2005;312:316–323. doi: 10.1124/jpet.104.073692. [DOI] [PubMed] [Google Scholar]
- Lees-Miller JP, Duan Y, Teng G.O, Duff HJ. Molecular determinant of high-affinity dofetilide binding to HERG1 expressed in Xenopus oocytes: involvement of S6 sites. Mol Pharmacol. 2000;57:367–374. [PubMed] [Google Scholar]
- Mitcheson JS, Chen J, Lin M, Culberson C, Sanguinetti MC. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci USA. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok NS, Lo YK, Tsui PT, Lam CW. Ketoconazole induced torsades de pointes without concomitant use of QT interval-prolonging drug. J Cardiovasc Electrophysiol. 2005;16:1375–1377. doi: 10.1111/j.1540-8167.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Nagatomo T, Fan Z, Ye B, Tonkovich GS, January CT, Kyle JW, et al. Temperature dependence of early and late currents in human cardiac wild-type and long Q-T ΔKPQ Na+ channels. Am J Physiol. 1998;275:H2016–H2024. doi: 10.1152/ajpheart.1998.275.6.H2016. [DOI] [PubMed] [Google Scholar]
- Rajamani S, Eckhardt LL, Valdivia CR, Klemens CA, Gillman BM, Anderson CL, et al. Drug-induced long QT syndrome: hERG K+ channel block and disruption of protein trafficking by fluoxetine and norfluoxetine. Br J Pharmacol. 2006;149:481–489. doi: 10.1038/sj.bjp.0706892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley JM, Milnes JT, Duncan RS, McPate MJ, James AF, Witchel HJ, et al. Inhibition of the HERG K+ channel by the antifungal drug ketoconazole depends on channel gating and involves the S6 residue F656. FEBS Lett. 2006;580:1999–2005. doi: 10.1016/j.febslet.2006.02.073. [DOI] [PubMed] [Google Scholar]
- Roden DM. Pharmacogenetics and drug-induced arrhythmias. Cardiovasc Res. 2001;50:224–231. doi: 10.1016/s0008-6363(00)00302-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Chapula JA, Navarro-Polanco RA, Culberson C, Chen J, Sanguinetti MC. Molecular determinants of voltage-dependent human ether-a-go-go related gene (HERG) K+ channel block. J Biol Chem. 2002;277:23587–23595. doi: 10.1074/jbc.M200448200. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Liu X, Xiong Q, Shikano S, Li M. Chronic inhibition of cardiac Kir2.1 and HERG potassium channels by celastrol with dual effects on both ion conductivity and protein trafficking. J Biol Chem. 2006;281:5877–5884. doi: 10.1074/jbc.M600072200. [DOI] [PubMed] [Google Scholar]
- Tonini M, De Ponti F, Di Nucci A, Crema F. Review article: cardiac adverse effects of gastrointestinal prokinetics. Aliment Pharmacol Ther. 1999;13:1585–1591. doi: 10.1046/j.1365-2036.1999.00655.x. [DOI] [PubMed] [Google Scholar]
- Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- Tsai WC, Tsai LM, Chen JH. Combined use of astemizole and ketoconazole resulting in torsade de pointes. J Formos Med Assoc. 1997;96:144–146. [PubMed] [Google Scholar]
- Venkatakrishnan K, Von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38:111–180. doi: 10.2165/00003088-200038020-00002. [DOI] [PubMed] [Google Scholar]
- Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999;354:1625–1633. doi: 10.1016/S0140-6736(99)02107-8. [DOI] [PubMed] [Google Scholar]
- Yao JA, Du X, Lu D, Baker RL, Daharsh E, Atterson P. Estimation of potency of HERG channel blockers: impact of voltage protocol and temperature. J Pharmacol Toxicol Methods. 2005;52:146–153. doi: 10.1016/j.vascn.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363–1372. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ramamoorthy Y, Kilicarslan T, Nolte H, Tyndale RF, Sellers EM. Inhibition of cytochromes P450 by antifungal imidazole derivatives. Drug Metab Dispos. 2002;30:314–318. doi: 10.1124/dmd.30.3.314. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, et al. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M, Duruz H, Guinand O, Broccard O, Levy P, Lacatis D, et al. Torsades de pointes after treatment with terfenadine and ketoconazole. Eur Heart J. 1992;13:1002–1003. doi: 10.1093/oxfordjournals.eurheartj.a060277. [DOI] [PubMed] [Google Scholar]