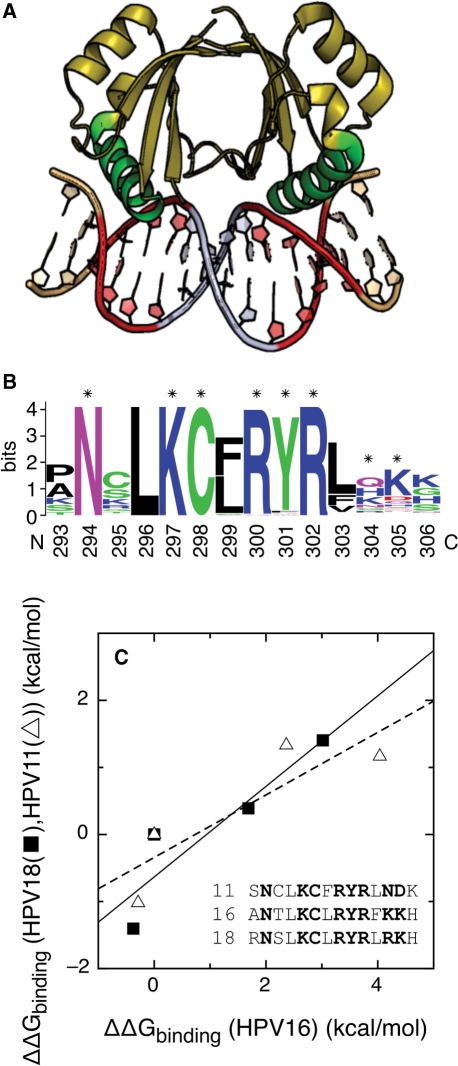
Figure 1.
Conserved features of the E2–DNA interaction. (A) Complex of the c-terminal domain of the HPV18 E2 protein with the idealized target DNA sequence CAACCGAATTCGGTTG. The two four-base half-sites in direct contact with the protein are shown in red, the four-base linker in silver and the two flanking bases in gold. The protein helices that contact the DNA directly in green. (B) Sequence logo (63,68) of the recognition helix for alpha papillomaviruses. Protein residues contributing more than 0.8 kcal/mol to the binding energy of HPV16 E2 (23) are indicated with asterisks. (C) Correlation between the free energies of binding of E2 proteins from HPV type 11 and 16 to four E2-BSs (open triangle) (16,19,20) and of E2 proteins from HPV types 18 and 16 to another set of four E2-BSs (filled square) (16,19,20). The correlation R-values are 0.87 (16/11 pair, dashed line) and 0.91 (16/18 pair, continuous line). The sequences of the DNA-binding helix of the three proteins are also shown, with the side chains contributing more than 0.8 kcal/mol to the binding energy of HPV16 E2 (23) in bold.