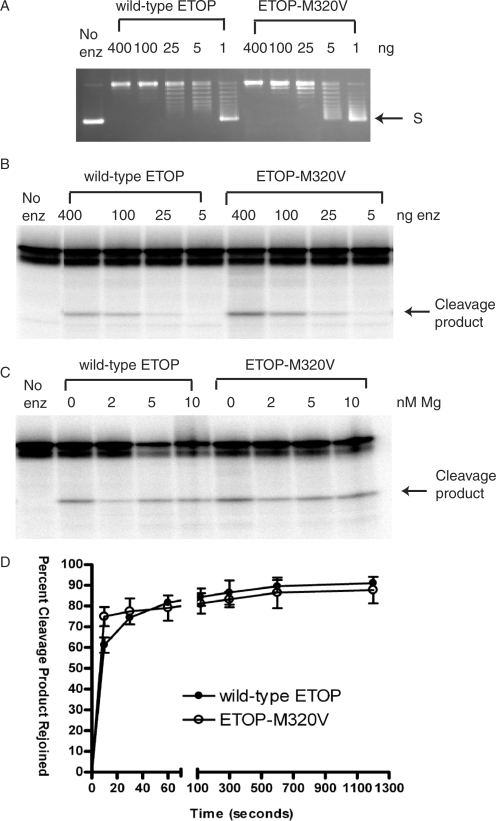
Figure 3.
The M320V mutation enhanced DNA cleavage by ETOP without affecting the relaxation and religation activities significantly. (A) Similar relaxation activity for wild-type ETOP and ETOP-M320V assayed in the presence of 6 mM MgCl2 at 37°C for 30 min. S: supercoiled plasmid DNA. (B) Comparison of cleavage of oligonucleotide substrate by wild-type ETOP and ETOP-M320V enzymes. The indicated amount of enzyme was incubated with 0.5 pmole of substrate for 30 min at 37°C in the absence of any MgCl2. (C) ETOP-M320V does not require magnesium for DNA cleavage. 400 ng of enzyme was incubated with 0.5 pmole of substrate at 37°C for 10 min. (D) DNA religation by ETOP-M320V mutant enzyme. The olignonucleotide substrate was incubated with 400 ng of enzyme for 10 min at 37°C in the absence of added MgCl2 before being placed on ice and the addition of 5 mM MgCl2 along with 1 M NaCl to promote DNA religation of the cleavage product and subsequent dissociation of the enzyme from the religated DNA. The reactions were allowed to proceed at 0°C for the indicated length of time before being stopped for analysis of the remaining cleavage product by gel electrophoresis.