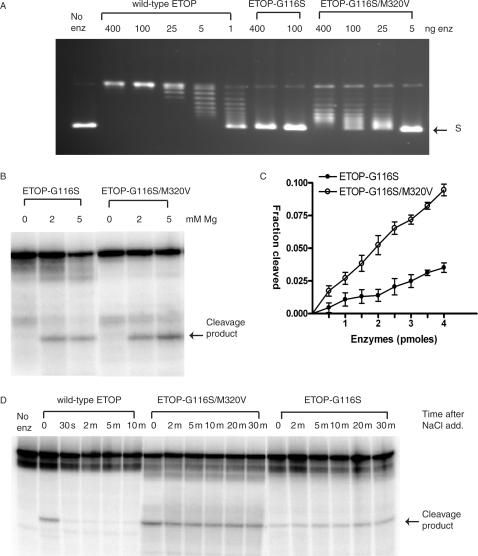
Figure 4.
Effect of addition of the M320V mutation to the ETOP-G116S mutant enzyme activities. (A) Restoration of a low level of relaxation activity to the ETOP-G116S mutant enzyme by the M320V mutation. The indicated amount of enzyme was incubated with supercoiled plasmid DNA in buffer containing 6 mM MgCl2 at 37°C for 30 min. S: supercoiled plasmid DNA. (B) Dependence of magnesium for DNA cleavage. 400 ng of enzyme was incubated with 0.5 pmole of substrate for 30 min at 37°C. (C) Quantitation of oligonucleotide (5 pmole) cleavage by ETOP-G116S (filled circles) and ETOP-G116S/M320V (open circles) enzymes in the presence of 5 mM MgCl2. The data plotted represents the average and standard deviation from three different experiments. (D) DNA religation inhibition for the ETOP-G116 and ETOP-G116S/M320V mutants. The oligonucleotide substrate was incubated with 400 ng of enzyme in the presence of 5 mM MgCl2 at 37°C for 10 min at 37°C before the addition of 1 M NaCl and further incubation at 37°C for the indicated length of time.