Figure 1.
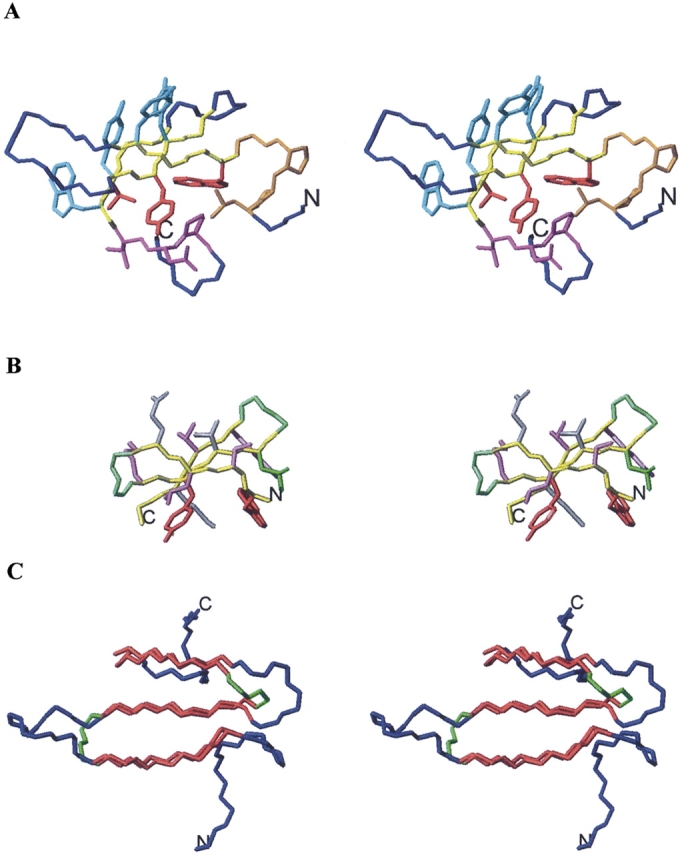
(A) Stereoscopic view of WW-P structure. Backbone atoms for β-strand residues aligned to Betanova, Betanova-LLM, and taken for the design of the chimeric peptides (Table 1) are shown in yellow. Backbone and side chain atoms for N-terminal and C-terminal residues incorporated in some of the chimeric peptides are shown in orange and magenta, respectively. Side chain atoms for β-strand residues common to WW-P and Betanova are indicated in red. Side chain atoms for β-strand residues incorporated in some of the designed peptides are shown in cyan. The remaining WW-P backbone atoms are colored in blue. (B) Stereoscopic view of Betanova-LLM structure. Side chain atoms for β-strand residues incorporated in all designed peptides are shown in purple. Side chain atoms for β-strand residues common to WW-P and Betanova are indicated in red. All displayed backbone atoms are included in the chimeric peptides. β-strand backbone atoms are shown in yellow and β-turn backbone atoms in green. (C) Stereoscopic view of WW-P (in blue) and Betanova-LLM (in green) backbone atoms superposed over β-strand residues (in red). N and C termini are labeled.
