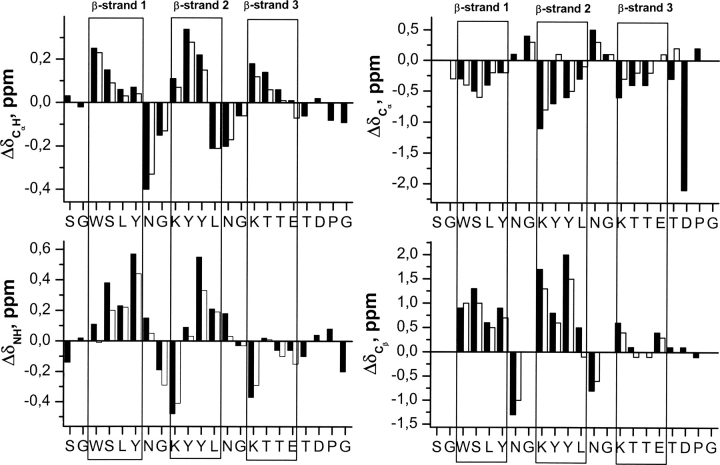
Figure 4.
Histograms of ΔδCαH (ΔδCαH = δCαH observed − δCαH random coil, ppm), ΔδNH (ΔδNH = δNH observed − δNH random coil, ppm), ΔδCα (ΔδCα = δCα observed − δCα random coil, ppm), and ΔδCβ (ΔδCβ = δCβ observed − δCβ random coil, ppm) values as a function of sequence for peptides 3SBWW-2 (filled bars) and Betanova-LYYL (open bars) at pH 5.5 and 10°C. Random coil values for the 1H chemical shifts of CαH protons and for the 13C chemical shifts of Cα and Cβ carbons were taken from Wishart et al. (1995). ΔδNH values were obtained by using the CSDb program (Fesinmeyer et al. 2005). N and C termini residues that may be affected by charge-end effects are not plotted. β-Strand regions are boxed.