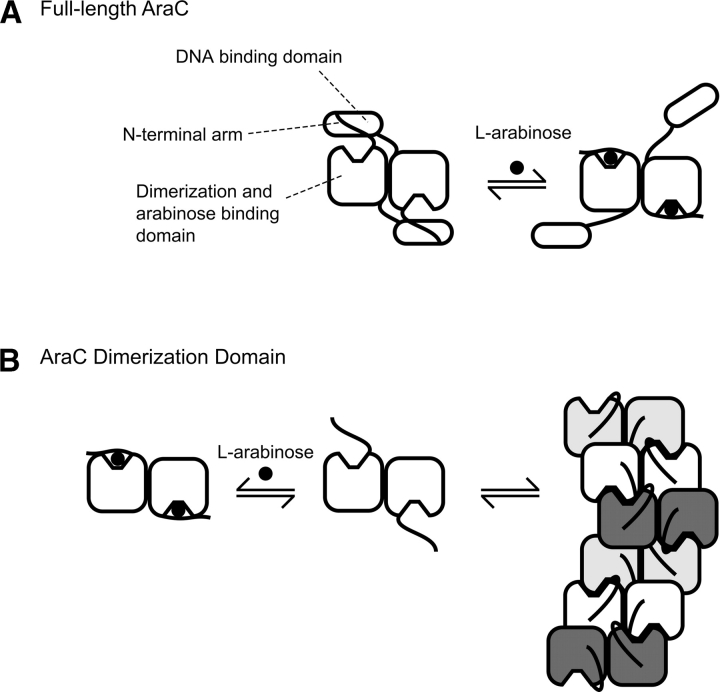
Figure 1.
Behavior of the full-length AraC protein (A) and the dimerization domain fragment of AraC (B) in the presence and absence of the ligand L-arabinose. For full-length AraC protein in the absence of arabinose, binding of the N-terminal arms to the DNA binding domains helps hold the domains fixed. As explained in the cited references, in vivo this favors DNA looping and repression of the nearby ara genes. When arabinose is present, the arms bind more tightly to the dimerization domains, thereby freeing the DNA binding domains, which ultimately results in induction of the nearby ara genes. AraC protein lacking the DNA binding domain; that is, dimerization domain in the absence of ligand forms indefinite-length oligomers through interactions involving the ligand-binding pocket as indicated.