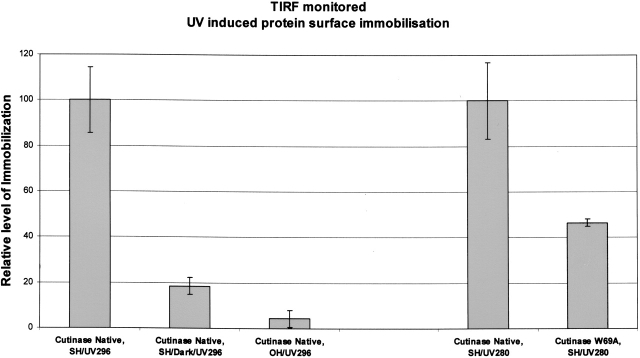
Figure 2.
TIRF data confirming UV (296 nm) induced immobilization of cutinase molecules on a thiol-derivatized quartz glass surface. When native cutinase was illuminated both with 296-nm and 280-nm UV light, data showed protein immobilization on thiol-derivatized surfaces (see Cutinase Native, SH/UV296 [first bar] and Cutinase Native, SH/UV280 [fourth bar]; 296 means that illumination was carried out with 296-nm light, which exclusively excites the single tryptophan; 280 means that illumination was carried out with 280-nm light, which excites all aromatic residues). Almost no protein immobilization was achieved when the protein was not being UV-illuminated while flowing on the thiolated (SH) slide surface (see Cutinase Native, SH/Dark/UV296 [second bar]; UV296 means that after flowing the protein on the slide surface, TIRF was monitored exciting the slide surface with 296-nm light). Also, almost no protein immobilization was achieved when using a bare (OH) quartz slide (non-thiolated slide) (see Cutinase Native, OH/UV296 [third bar]), despite the fact that the protein was being UV-illuminated with 296-nm light while flowing on the slide. UV illumination of native and W69A mutant cutinase (Trp-depleted mutant) with 280-nm light revealed UV-induced immobilization of the proteins to a different extent: Native cutinase showed an approximately two times larger extent of light-induced immobilization onto thiolated slides (cf. Cutinase Native, SH/UV280 [fourth bar] and Cutinase W69A, SH/UV280 [fifth bar]).