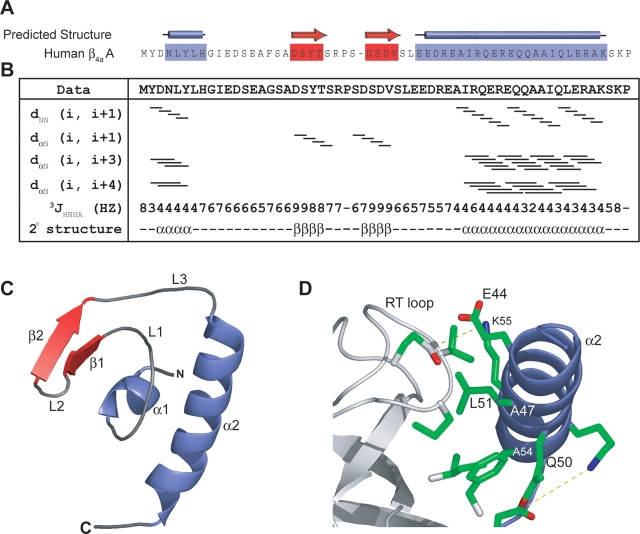
Figure 1.
(A) Secondary structure predictions performed with the PSA server (http://bmerc-www.bu.edu/psa; White et al. 1994) indicate that the β4a-A domain has a mixture of α-helix and β-sheet. (B) Secondary structural characterization by multidimensional NOESY-HSQC and HNHA J coupling experiments. Solid bars for dNN (i, i + 1) and dα N (i, i + 1) represent continuous cross peaks observed in 15N-edited NOESY-HSQC experiments run with a 50-msec mixing time. Lines for dα N (i, i + 3) and dα N (i, i + 4) represent NOE cross-peaks between residues three and four amino acids away, respectively. The 3JHNHA coupling constants provide further information on the secondary structure boundaries. These results outline the secondary structural elements present in the β4a-A domain and correlate with predicted structural elements. (C ) Solution structure of the β4a-A domain determined with a backbone RMSD of 0.73 A °(Table 1). The β4a-A domain is independently folded with the first helix (α 1) coordinating two β-strands (β1 and 2), three loops (L1–3), and a C-terminal α-helix (α 2) to create a globular, hydrophobic core. (D) The α 2 helix is involved in packing against the SH3 domain (Opatowsky et al. 2004). Conserved residues are involved in hydrophobic packing (A54, L51, and A47 on the A domain, and V80, F100, W104, and I106 on the SH3 domain) and formation of salt bridges (R53–E97 and K55–E115) between the A and the SH3 domains of β4a.