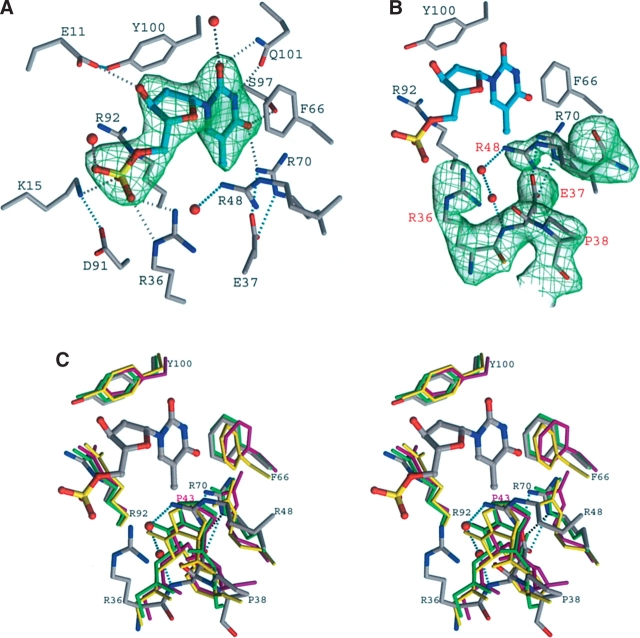
Figure 2.
TMP-binding site of SaTMK. (A) Simulated annealing omit map of bound TMP in his-tagged SaTMK structure. Carbon atoms of TMP are drawn in cyan; those of residues interacting with TMP are drawn in gray. Potential hydrogen bonds are indicated by dotted lines; those that directly involve atoms of the substrate are drawn in gray, others in cyan. (B) Simulated annealing omit map of the (E/F)P loop and Arg 48 in his-tagged SaTMK structure. Carbon atoms of TMP are drawn in cyan; those of residues of SaTMK are drawn in gray. (C) Stereo view of the superposition of the TMP-binding site of SaTMK, EcTMK, MtTMK, and human TMK. The important residues defining the TMP-binding site and the positions of the conserved cis-proline are shown. The residues of SaTMK and TMP are drawn in atom colors, while the residues of EcTMK, MtTMK, and human TMK are drawn in green, yellow, and magenta, respectively. The residues of SaTMK are labeled in black, and the cis-Pro 43 of human TMK is labeled in magenta. Interaction of Arg 48 with the main-chain N atom of Glu 37 via water molecules and the interaction of Glu 37 with Arg 70 are indicated by cyan dotted lines. The interaction of Glu 40 with Arg 74 of EcTMK is indicated by black dotted lines.