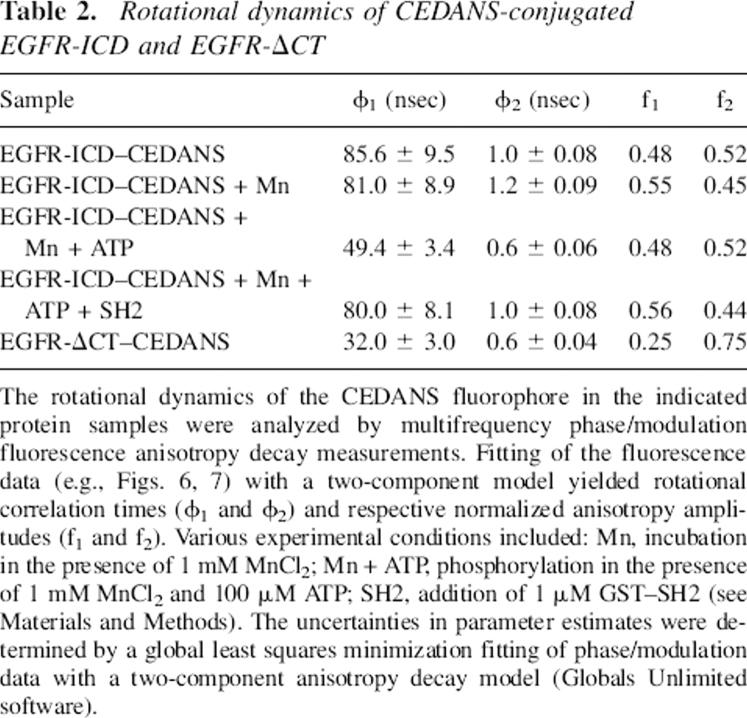
Table 2.
Rotational dynamics of CEDANS-conjugated EGFR-ICD and EGFR-ΔCT
The rotational dynamics of the CEDANS fluorophore in the indicated protein samples were analyzed by multifrequency phase/modulation fluorescence anisotropy decay measurements. Fitting of the fluorescence data (e.g., Figs. 6, 7) with a two-component model yielded rotational correlation times (ϕ1 and ϕ2) and respective normalized anisotropy amplitudes (f1 and f2). Various experimental conditions included: Mn, incubation in the presence of 1 mM MnCl2; Mn + ATP, phosphorylation in the presence of 1 mM MnCl2 and 100 μM ATP; SH2, addition of 1 μM GST–SH2 (see Materials and Methods). The uncertainties in parameter estimates were determined by a global least squares minimization fitting of phase/modulation data with a two-component anisotropy decay model (Globals Unlimited software).