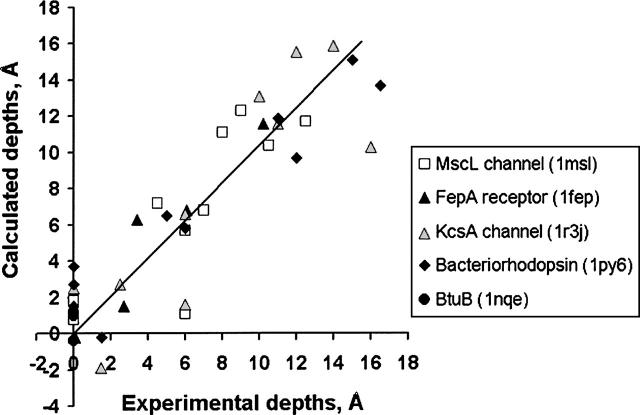
Figure 2.
Comparison of calculated and experimental membrane penetration depths of spin-labeled Cys residues in TM proteins. Five TM proteins were studied: MscL channel (1msl, open square), FepA receptor (1fep, black triangle), KcsA channel (1r3j, gray triangle), bacteriorhodopsin (1py6, black diamond), and BtuB porin (1nqe, black circle). The experimental distances are taken from the original publications (Altenbach et al. 1990, 1994; Greenhalgh et al. 1991; Klug et al. 1997; Perozo et al. 1998, 2001; Fanucci et al. 2002) but counted relative to the depth where parameter Φ is equal to zero. Points with zero depth correspond to residues that have been identified as first or last in the membrane-embedded segments of α-helices of β-strands. Calculated depths are defined as distances from Cβ-atoms of the corresponding residues to the closest boundary plane.