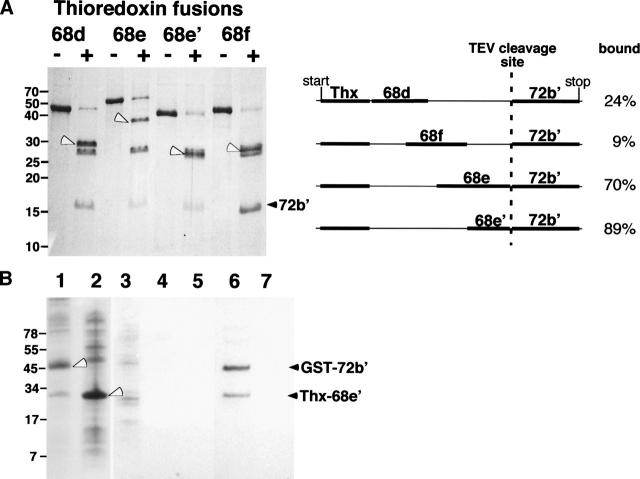
Figure 6.
Binding of the N-terminal region of SRP72 to fragments of SRP68. (A) Fragments 68d, 68e, 68e′, 68f, fused to thioredoxin, and 72b′, separated on a 15% polyacrylamide Tris-glycine gel. Lanes labeled with plus signs indicate TEV protease digested samples. The black arrowhead marks the liberated 72b′ fragment; white arrowhead indicates the various SRP68 fragments fused to thioredoxin. Weak bands in the 28-kDa range correspond to the TEV protease. The right panel outlines the four fusion constructs indicating the start and stop codons of the plasmid as well as the position of the TEV cleavage site. Analysis of the TEV-cleaved fusion proteins by sucrose gradient centrifugation followed by the SDS-PAGE of the fractions showed degrees of binding as expressed in percent bound/free 72b′. (B) Pull-down assays with GST–72b′ and Thx–68e′. E. coli lysates analyzed on 10% Tris-Tricine SDS gels containing expressed GST–72b′ (lane 1) and Thx–68e′ (lane 2). (Lanes 3–5) Proteins in the wash of the GST-Sepharose column; (lane 6) coelution of GST–72b′ and Thx–68e′ using 20 mM glutathione; (lane 7) control reaction lacking GST–72b′ proving that Thx–68e′ has no intrinsic affinity for GST-Sepharose.