Figure 1.
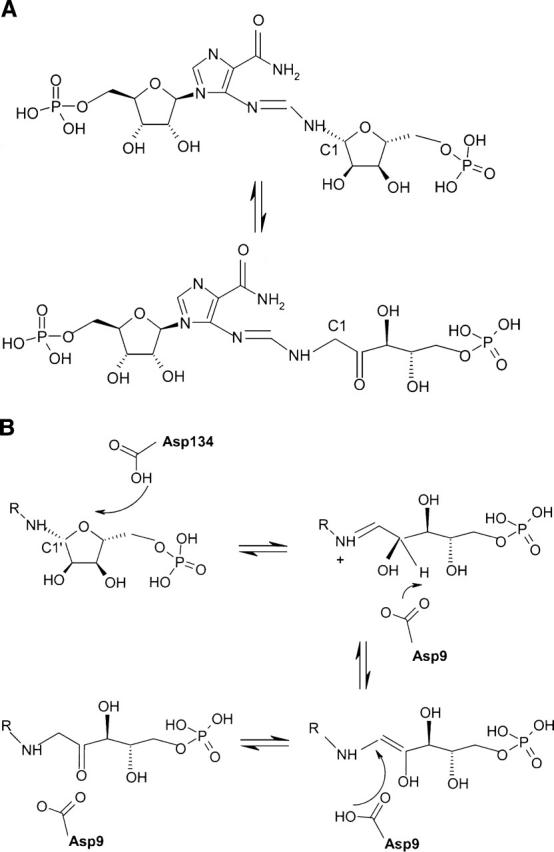
(A) Reaction catalyzed by His6: ProFAR (N-(5′-phospho-D-ribosylformimino)-5-amino-1-(5″-phosphoribosyl)-4-imidazolecarboxamide) ⇆ PRFAR (N-(5′-phospho-D-1′-ribulosylformimino)-5-amino-1-(5″-phosphoribosyl)-4-imidazolecarboxamide. (B) Proposed reaction mechanism of His6. The substrate binds as the closed form and ring opening is catalyzed by protonation mediated by Asp134 yielding the Shiff's base. After opening of the ring, the substrate is repositioned in front of the catalytic Asp9. This aspartate abstracts a proton from the C2′carbon and transfers it to the C1′position yielding the ribulose product.
