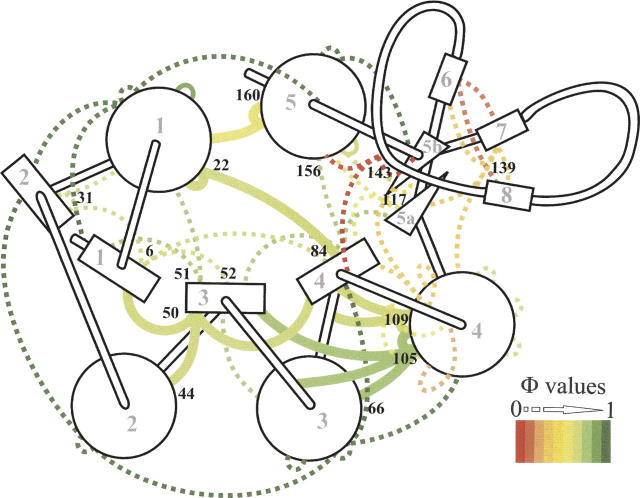
Figure 6.
Apoflavodoxin secondary structure cartoon showing new (continuous thick lines) and previously reported (Campos et al. 2004a) (discontinuous thin lines) ϕ-values reporting on the integrity of native side-chain interactions in the equilibrium thermal unfolding intermediate at 317.3 K. The native-like region of the intermediate is formed by the packing of helices 1–5, and strands 1–4 (where the ϕ-values are >0.6) and possibly strand 5a. In contrast, the long loop splitting strands 5a and 5b (which contains a small three-stranded b-sheet comprising strands 6, 7, and 8), and two additional loops, are markedly weakened (all ϕ-values <0.4) (Campos et al. 2004a). The ϕ-values are colored from dark green (native interactions) to red (lost interactions). ID numbers of helices (circles) and strands (rectangles) are marked in light gray, and the positions analyzed in this work are indicated in black.