Figure 3.
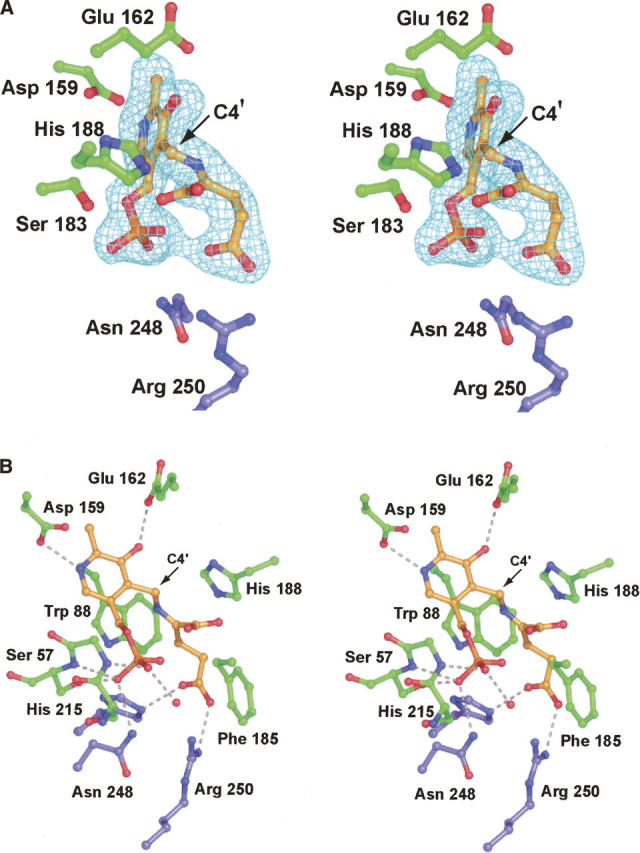
The structure of ColD with a glutamate ketimine intermediate trapped in the active site. Electron density corresponding to the ketimine intermediate is presented in A. The map was calculated in the same manner as described for Figure 2B. Note that the α-carboxylate group of the intermediate is not well ordered, suggesting free rotation. A close-up view of the active site is displayed in B. Possible hydrogen bonding interactions are indicated by the dashed lines. For the sake of clarity, the hydrogen bonding interaction between Ser 183 and the phosphoryl group of the ketimine intermediate was omitted. Ser 183 forms the same interaction with the ketimine phosphoryl group as it does with hydrated PLP (Fig. 2C).
