Figure 2.
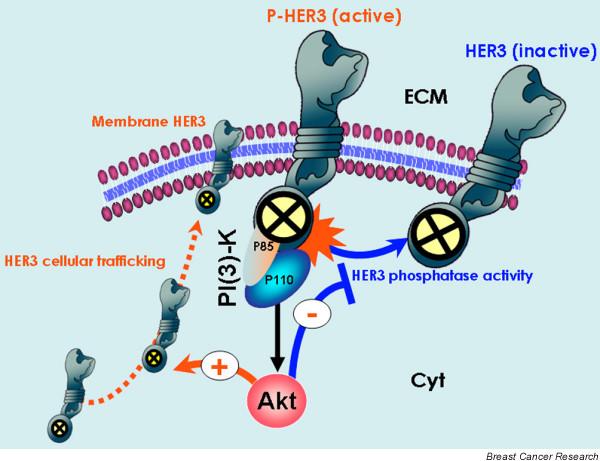
Biological markers to assess the efficacy of HER TKIs (I): Akt-regulated HER3 phosphorylation-dephosphorylation equilibrium. The study conducted by Sergina and coworkers [6] suggests that the biological marker used to assess the efficacy of human epidermal growth factor receptor (HER) tyrosine kinase inhibitors (TKIs) should be transphosphorylation of HER3 rather than autophosphorylation of HER1 or HER2 [6]. They reported that HER3 and phosphatidylinositol-3-OH kinase (PI [3]K)/Akt signalling is not effectively inhibited by current HER TKIs. Through Akt-mediated negative feedback signalling, a compensatory shift occurs in the HER3 phosphorylation-dephosphorylation equilibrium, driven by increased HER3 membrane expression (phosphorylation reaction) and by reduced HER3 phosphatase activity (dephosphorylation reaction). Despite significant inhibition of HER2 activity, the reactivation of HER3 signalling to a new steady-state HER3 phosphorylation level requires much higher concentrations of HER TKIs because the un-inhibited HER3 phosphorylation state is significantly higher in this new steady state. Therefore, although kinase-inactive HER3 is not a direct target of HER TKIs, HER3 substrate resistance appears to undermine their efficacy significantly.
