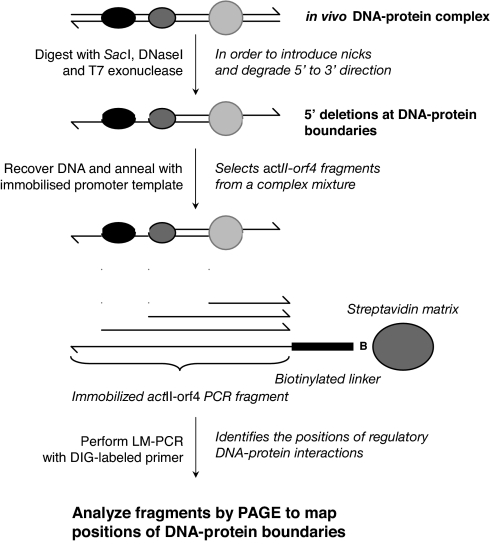
Fig. 1.
Schematic overview of the protocol for in vivo T7 exonuclease/DNase I mapping. To map the in vivo boundaries of DNA–protein complexes at specific locations within the S. coelicolor genome, DNase I and T7 exonuclease were added to freshly harvested cells. The DNase I introduced “nicks” into the DNA surrounding the complexes that then served as substrates for the 5′ to 3′ exonuclease activity of T7 exonuclease. DNA was recovered from the treated cells, and fragments from the targeted actII-orf4 promoter were captured by hybridization to an immobilized strand of a PCR fragment of the promoter that incorporated a biotinylated linker. Boundaries within the population of captured fragments were mapped by performing a PCR with a second DIG-labeled primer that hybridized to the complementary strand of the biotinylated linker. The sizes of the labeled products were determined by PAGE and chemoluminescent detection. Boundaries on the opposite strand were mapped in a similar manner, using a PCR product with the biotinylated linker at the opposite end.