Abstract
The glomerular filtration barrier prevents large serum proteins from being lost into the urine. It is not known, however, why the filter does not routinely clog with large proteins that enter the glomerular basement membrane (GBM). Here, we provide evidence that an active transport mechanism exists to remove immunoglobulins that accumulate at the filtration barrier. We found that FcRn, an IgG and albumin transport receptor, is expressed in podocytes and functions to internalize IgG from the GBM. Mice lacking FcRn accumulated IgG in the GBM as they aged, and tracer studies showed delayed clearance of IgG from the kidneys of FcRn-deficient mice. Supporting a role for this pathway in disease, saturating the clearance mechanism potentiated the pathogenicity of nephrotoxic sera. These studies support the idea that podocytes play an active role in removing proteins from the GBM and suggest that genetic or acquired impairment of the clearance machinery is likely to be a common mechanism promoting glomerular diseases.
Keywords: Fc receptor, glomerulonephritis
The filtration barrier of the glomerulus is composed of fenestrated endothelial cells, the glomerular basement membrane (GBM), and the foot processes and slit diaphragms of the podocytes (1). These three components work together to form a size- and charge-selective filter that blocks the passage of cells and larger proteins into the urine. The exact mechanism of filtration has been an active area of research for the last 50 years.
The most widely accepted model is that the glomerular filtration barrier functions as a series of sieves with increasing size selectivity (2, 3). The fenestrations of the endothelial cell function as a coarse filter blocking the entry of blood components that are larger than the fenestrae (≈70–100 nm in diameter). The GBM is thought to function as the main filter with a small pore size and the anionic charge formed of a lattice of collagen fibrils and polyanionic proteoglycans blocking the entry of most large proteins (4). Lastly, the podocyte foot processes and associated slit diaphragms are thought to function as a fine filter, setting an upper size limit of ≈70 kDa for proteins to enter the primary urinary ultrafiltrate (5, 6). The importance of the slit diaphragm is supported by the fact that genetic deficiencies of proteins required for slit diaphragm integrity, such as nephrin (7), lead to proteinuria and renal failure.
This model, however, does not explain why proteins at or larger than the exclusion limit of the podocyte slit diaphragms do not routinely accumulate behind the filter. To explain this conundrum, Smithies (8) proposed that the GBM functions like a chromatography gel. In his model, the GBM is variably permeable to proteins, being highly permeable to small proteins and less permeable to large proteins. This differential permeability can largely account for the size selectivity of the filtration apparatus, but assumes that the slit diaphragm is not a barrier to large proteins and only controls fluid flow. However, even in this model, significant amounts of abundant serum proteins such as albumin and IgG would be expected to enter the GBM and, following the direction of fluid flow, would accumulate behind the slit diaphragms of the podocyte. Consistent with this idea, proteins are known to accumulate under the foot processes of the podocyte in many disease states (9, 10). These observations suggest that proteins are normally cleared from the slit diaphragm and that this process may be impaired in certain disease states.
Because the podocytes form the final barrier to glomerular filtration, we hypothesized that they possess an active transport system to clear proteins that would otherwise clog the slit diaphragm. In a search for specific receptors that could mediate this process, we discovered that the IgG/albumin transport receptor, FcRn, was expressed in podocytes. FcRn was first discovered as the intestinal epithelial receptor responsible for transporting maternal IgG into the bloodstream of the baby (11–13). It was later found to be important for the long serum half-life of IgG (14–17). Recently, it was shown that FcRn also binds to albumin and presumably, by blocking its degradation, extends its serum half-life (18). FcRn is present in intracellular endosomes, where it intercepts internalized IgG and recycles it to the cell surface (19). In the absence of FcRn, internalized IgG traffics to the lysosome and is degraded. Consequently, FcRn-deficient mice exhibit short serum half-lives and low resting levels for both IgG and albumin (17, 18).
In this study, we investigated the expression of FcRn in the podocyte. Consistent with a role for FcRn in trafficking IgG across the filtration barrier, we found that FcRn-deficient mice had an impaired clearance of IgG from the GBM that resulted in the accumulation of IgG in the glomeruli of FcRn knockout mice as they aged. Impairing the clearance of protein from the GBM by administering excess protein resulted in increased sensitivity to nephrotoxic damage. Therefore, our results suggest that genetic or acquired impairment of the clearance mechanism may contribute to the pathology of glomerular disease.
Results
The IgG Transport Receptor FcRn Is Expressed in Mouse Podocytes.
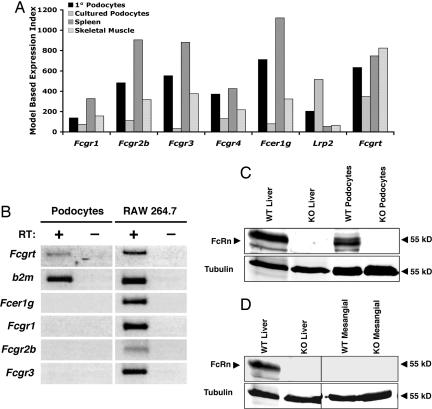
We hypothesized that podocytes use an active process to clear protein from the GBM. Because the major serum proteins are albumin and Ig, we investigated the expression of IgG and albumin receptors in primary and cultured podocytes by microarray analysis. We found that freshly isolated primary mouse podocytes expressed the transcript for the low-affinity Fcγ receptors (FcγRIIB and FcγRIII) in addition to the transcript for the albumin receptor megalin (Fig. 1A). In contrast, cultured podocytes expressed transcripts for megalin, but expressed much lower levels of the transcript for the Fcγ receptors. Both primary and cultured podocytes expressed the transcript for the IgG and albumin transport receptor, FcRn. Consistent with reports in human podocytes (20), we confirmed FcRn expression in cultured podocytes by both RT-PCR (Fig. 1B) and immunoblotting (Fig. 1C). In contrast to podocytes, primary mesangial cell cultures did not express FcRn (Fig. 1D).
Fig. 1.
FcRn is expressed in murine podocytes. (A) Affymetrix genechip data were analyzed by using dChip software (43). The model-based expression index indicates relative expression levels of the indicated genes across tissues. For comparison, expression data for spleen and skeletal muscle were obtained from a published dataset (43) deposited in the public Gene Expression Omnibus (GEo) database (www.ncbi.nlm.nih.gov/geo/). The gene symbols are as follows: FcγRI, Fcgr1; FcγRIIB, Fcgr2b; FcγRIII, Fcgr3; FcγRIV, Fcgr4; Fcγ chain, Fcer1g; Megalin, Lrp2; and FcRn, Fcgrt. (B) RT-PCR of cDNA from podocytes differentiated for 10 days at 37°C is shown for FcRn (Fcgrt) and its obligate light chain, β2-microglobulin (b2m). cDNA from the mouse macrophage cell line RAW 264.7 is shown as a positive control. RT-PCR for genomic DNA contamination [without reverse transcriptase (RT)] was negative. Results are representative of two different experiments. (C) Two hundred twenty-five micrograms of postnuclear lysis supernatant from FcRn wild-type and knockout podocytes was immunoblotted with affinity-purified rabbit anti-FcRn serum. Liver lysates from FcRn wild-type and knockout mice were immunoblotted as positive and negative controls. Protein loading was detected by immunoblotting for α-tubulin. (D) Two hundred twenty-five micrograms of postnuclear lysis supernatant from FcRn wild-type and primary mesangial cells was immunoblotted with affinity-purified rabbit anti-FcRn serum. Expression and loading controls are as described in Fig. 1C.
Decreased IgG Clearance in FcRn-Deficient Mice.
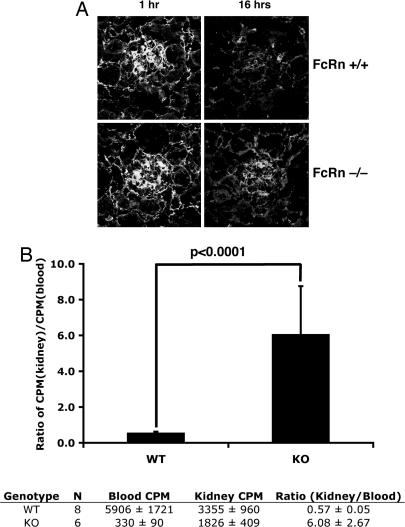
Because FcRn has been shown to function in epithelial cells as a transcytosis receptor (21, 22), it was an attractive candidate to transport IgG from the GBM into the urinary space. FcRn also is highly expressed in the proximal tubule (20, 23), where it can reabsorb the IgG and albumin that pass through the glomerulus (24). To test whether podocytes use FcRn to clear IgG from the GBM, we measured the clearance of IgG from the kidneys of wild-type versus FcRn-deficient mice in vivo. To do this, we first injected wild-type or FcRn knockout mice with 1 mg of human IgG intravenously and then measured IgG retention in the kidneys by immunofluorescence. One hour after injection, human IgG was predominantly localized to glomeruli, and there was no difference in IgG retention between wild-type and knockout mice (Fig. 2A). However, 16 h after the injection, FcRn knockout mice stained strongly for human IgG in their glomeruli compared with wild-type mice (Fig. 2A). To quantitate this result, we injected I125-labeled human IgG into wild-type and FcRn knockout mice. Kidneys were harvested after 1 week, and the associated radioactivity was measured. In wild-type animals, FcRn expression in the vascular endothelium functions to recycle internalized IgG back into the circulation, protecting it from catabolism (23, 25). In knockout animals, internalized IgG is degraded in lysosomes (19). Consequently, the half-life of IgG is significantly shorter in FcRn-deficient mice (17). We therefore normalized the radioactivity in the kidney to the amount of radioactive IgG in the blood to compensate for the lower levels of serum IgG after 1 week in knockout animals. These measurements showed that FcRn knockout mice retained proportionately more labeled IgG in their kidneys compared with wild-type animals (Fig. 2B). These experiments suggested that the IgG that enters the filtration barrier is not cleared as efficiently in FcRn-deficient mice as compared with wild-type mice and supports the idea that FcRn plays an active role in clearing IgG from the GBM.
Fig. 2.
FcRn-deficient mice have a reduced ability to clear IgG from the GBM. (A) FcRn wild-type and knockout mice were injected with 1 mg of human IgG intravenously. Then, 1 or 16 h after injection, kidneys were harvested and stained for human IgG. (B Upper) One week after the injection of I125-labeled IgG, counts in whole decapsulated kidneys were normalized to TCA-precipitable radioactive counts from 50 μl of serum. (B Lower) Raw counts are shown. Blood cpm represents TCA-precipitable radioactivity from 50 μl of serum, and kidney cpm represents counts from whole unperfused, decapsulated kidney. P < 0.0002 by two-tailed Student's t test. Results are the combined results from two independent experiments using age- and sex-matched mice with similar results.
FcRn Deficiency Results in Increased Glomerular IgG Retention.
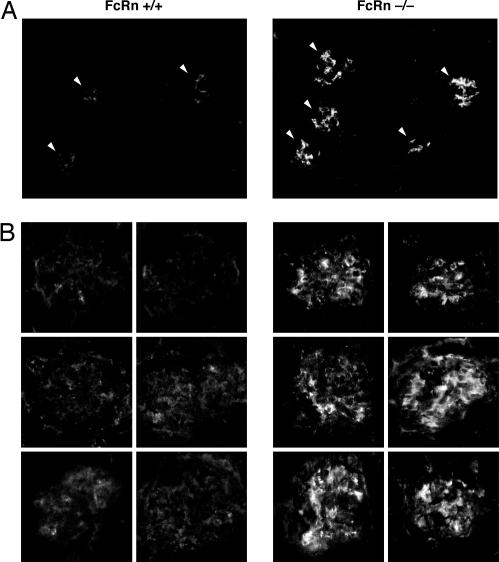
To test the long-term consequences of FcRn deficiency on the clearance of IgG from the GBM, we harvested kidneys from wild-type and FcRn-deficient mice up to 6 months of age and stained for mouse IgG. We consistently noted increased IgG staining in FcRn-deficient animals compared with wild-type animals as young as 2.5 months of age (data not shown). The difference, however, was most apparent in 6-month-old animals (Fig. 3). The pattern of glomerular IgG localization was mostly mesangial, but capillary loop staining also was readily observed. This increased IgG accumulation in FcRn-deficient mice was observed despite the fact that, in the absence of FcRn, the serum IgG concentration in these mice is only 10–20% of the normal level (17). Thus, the absence of FcRn leads to the glomerular accumulation of IgG with age.
Fig. 3.
FcRn-deficient mice accumulate glomerular IgG with age. Kidneys from FcRn wild-type and knockout mice were stained for mouse IgG retention at 6 months of age. The results are representative of two different experiments with six to eight age-matched mice per group. (A) Low-power (×200) view of IgG staining from a representative wild-type and FcRn-deficient mouse. Arrowheads indicate individual glomeruli. (B) High-power (×630) view of IgG staining. Panels represent typical glomerular IgG staining from six mice in each group (one panel per mouse).
A Protein Load Increases the Sensitivity of Mice to Serum-Induced Nephritis.
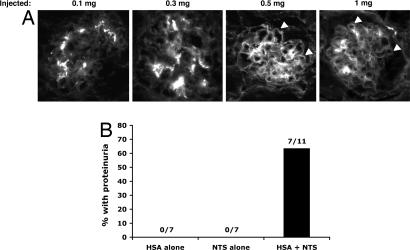
Next, we asked whether the protein-handling capacity of the glomerulus could be saturated by administering increasing doses of IgG to mice. Because it is difficult to control serum IgG levels in FcRn knockout mice because they rapidly catabolize injected IgG (17), we used FcRn wild-type mice for the saturation experiments. We observed that the accumulation of IgG in kidneys of mice was dose-dependent because we detected increasing IgG accumulation in the GBM only at the highest doses of purified mouse IgG (Fig. 4A). We postulated that protein overload might impair the clearance of pathogenic agents that become trapped in the GBM and thereby enhance their pathogenic effect. To test this hypothesis, we used a previously characterized nephrotoxic antiserum (26) at a dose that had little to no effect on 4-month-old wild-type animals. This dose of nephrotoxic antibody was then injected into animals that had previously been injected i.p. with albumin or PBS or were left uninjected. Although none of the uninjected or PBS-injected animals developed proteinuria after injection with the nephrotoxic antibody, a significant fraction (≈63%) of animals injected with albumin did (Fig. 4B). This finding suggested that the accumulation of protein in the GBM impairs the overall clearance of proteins that enter the GBM, enhancing the ability of nephrotoxic agents to inflict injury.
Fig. 4.
Saturating the glomerular clearance mechanism increases the pathogenicity of nephrotoxic serum. (A) Injection of increasing doses of purified mouse IgG i.v. leads to glomerular retention of mouse IgG in 6-month-old wild-type mice only at higher doses. Capillary loop staining is indicated by arrowheads. (Magnification: ×200.) (B) A protein bolus sensitizes mice to nephrotoxic nephritis. HSA alone, mice were injected with two doses of HSA alone; NTS alone, mice were injected with a low dose of nephrotoxic serum (NTS) alone; HAS + NTS, mice were injected with a low dose of NTS after injection of HAS (see Experimental Procedures for injection protocol). The protein content of urine for all mice in HSA alone and NTS alone groups was <30 mg/dl. Six of seven mice in the HAS + NTS group had proteinuria of >3,000 mg/dl, and one mouse had proteinuria of >300 mg/dl. The four remaining mice in this group had <30 mg/dl of protein in their urine. Values are the combined results from two independent experiments with similar results and were significant (P ≤ 0.002) by Fisher's exact test.
Discussion
Current models propose that the glomerular filtration barrier consists of a fenestrated endothelium, the GBM, and finally the slit diaphragm of the podocytes. The charge selectivity of the glomerular filtration barrier resides at the level of the charged glycocalyx of the endothelium and the negatively charged matrix of the GBM (27, 28). Although this charge barrier may repel a significant amount of serum protein, proteins that do enter the GBM are directed toward the podocyte slit diaphragm by fluid flow and diffusion (8). Assuming that the slit diaphragm is not freely permeable to protein, large serum proteins that enter the GBM should accumulate behind this final component of the filter. Why, then, doesn't the kidney filter routinely clog with abundant endogenous serum proteins such as albumin and IgG?
Even assuming very low permeability coefficients for albumin and IgG into the GBM, given the high blood flow to the kidney, podocytes would still be expected to encounter significant quantities of these proteins each day. Consequently, internalization and degradation of this protein would be maladaptive. Rather, receptor-mediated transcytosis of protein into the urinary space, followed by reabsorption in the proximal tubule, would solve two problems. First, transcytosis of protein accumulated at the filtration slit would serve to keep the basement membrane and slit diaphragms clear of protein. Second, reabsorption of proteins by the proximal tubule would prevent loss of transcytosed protein into the urine. We therefore hypothesized that podocytes use an active clearance mechanism to keep the GBM and the slit diaphragms clear of protein. In RNA microarray studies, we found only one transcytotic receptor that satisfied these criteria, the epithelial transport receptor, FcRn. We confirmed that FcRn was expressed in podocytes by RT-PCR and immunoblotting.
Because FcRn is expressed mainly in intracellular vesicles, how does it encounter IgG? It is conceivable that in vivo podocytes use the classical FcγRs and megalin to internalize protein. Consistent with this idea, a recent report describes the receptor-mediated uptake of IgG into intracellular vesicles of cultured murine podocytes (29). Once internalized, FcRn can bind IgG in an acidic intracellular endosome and transport it to the urinary space. Previous work has shown that primary rat podocytes return internalized IgG intact back to the cell surface rather than degrading it (30). Thus, the expression of FcRn in podocytes provides a mechanism to clear IgG from the GBM and deliver it intact into the urinary space.
Consistent with this mechanism, FcRn-deficient mice showed reduced clearance of radioactive IgG from their kidneys. Also, as they aged, FcRn-deficient mice had increased IgG deposition in their glomeruli. This accumulation was particularly striking given the low constitutive levels of IgG in the FcRn-deficient mice. Because of its increased turnover, the resting IgG level in FcRn-deficient mice is only ≈10–20% of the normal level (17). Perhaps because of these low serum IgG levels, FcRn-deficient mice did not become proteinuric as they aged (data not shown). This fact suggested that the extrarenal effects of FcRn deficiency may have compensated for the lack of FcRn function in the kidney. The generation of animals that specifically lack FcRn expression only in the podocyte will be necessary to separate the systemic from the renal contributions of FcRn. We would predict that in these animals, serum IgG levels would be normal, but the lack of FcRn in the podocyte would accelerate IgG accumulation in the glomerulus, leading to pathological changes.
Of the cells types present in the glomerulus, it was possible that the mesangial cell or the podocyte might be clearing IgG and albumin from the GBM. Although the pattern of IgG accumulation in FcRn-deficient mice was predominantly mesangial, our results suggest that the podocyte is mainly responsible for clearing protein that is trapped at the slit diaphragms. First, FcRn has been previously reported to be expressed in human podocytes, but not in mesangial cells (20), and our studies confirmed FcRn expression in mouse podocytes, but not in mesangial cells. Second, the podocyte slit diaphragms constitute the final barrier to glomerular filtration, and thus the podocyte is strategically positioned to transport proteins that are trapped at this barrier. Third, further downstream in the nephron, FcRn is strongly expressed in the proximal tubule (20, 23), where it can reabsorb glomerularly filtered IgG (24). This mechanism has already been suggested for albumin, which also binds to FcRn (18, 31–33). For these reasons, we chose to focus on the podocyte's mechanism to clear IgG from the GBM via FcRn.
How much protein reaches the tubular system after glomerular filtration? Normally, the fractional clearance of serum macromolecules >70 kDa is very low. Estimates of the fractional clearance of albumin obtained from micropuncture or isolated kidney perfusion studies are in the range of 0.062% (34) to 0.15% (35). However, even with a low fractional clearance of 0.062%, >4 g of albumin would reach the tubules per day in humans (8). Therefore, tubular reabsorption of albumin and IgG by FcRn would be necessary to prevent the loss of significant quantities of protein into the urine.
Rapid clearance of IgG and immune complexes from the GBM would prevent fixation of complement and is likely to be important in preventing immune complex-mediated renal damage. In experimental models of immune complex disease, podocytes can internalize immune complexes trapped in the GBM (2, 36–40). Based on the expression of IgG receptors on podocytes, the handling of IgG and immune complexes is likely to be part of the normal function of podocytes. Only when the podocyte clearance mechanism is saturated would there be an increased susceptibility to antibody-mediated damage. Consistent with this idea, we showed that overloading the clearance capacity of the kidney with excess protein increased the toxicity of nephrotoxic antibodies. In a similar manner, increased deposition of immune complexes in chronic diseases such as systemic lupus erythematosus may overwhelm the clearance mechanism of podocytes and result in immune complex accumulation with adverse consequences.
Taken together, our results suggest a model for glomerular filtration. The components of the glomerular filter (the endothelium, the GBM, and the podocyte slit diaphragm) act together to largely exclude serum macromolecules from the primary urine. However, a significant daily protein load of albumin and IgG would still be expected to be trapped at the podocyte slit diaphragm. Without a mechanism of clearance, this protein would block the glomerular filter. Podocytes can use specific receptors or possibly even nonspecific mechanisms to internalize this protein from the slit diaphragm. We have demonstrated one mechanism involving FcRn that could transcytose IgG around the slit diaphragm into the urinary space. Then, downstream, IgG can be reabsorbed by FcRn in the proximal tubule. Protein accumulation in the glomerulus therefore reflects a balance between deposition and active clearance of proteins from the GBM mediated at least in part by podocytes. When this clearance mechanism is saturated, either through increased protein deposition or genetic deficiency in components of the clearance mechanism, there is an increased susceptibility to glomerular injury.
Experimental Procedures
Mice.
C57BL/6J and 129 × 1/J mice at various ages were purchased from The Jackson Laboratory. FcRn-deficient mice on a C57BL/6 background were generated at The Jackson Laboratory (17) and have been backcrossed onto the C57BL/6J background for >13 generations. All mice were housed at specific pathogen-free facilities at Washington University and used in accordance with institutional animal care and use committees.
Protein Preparation and Injections.
Indicated volumes of rabbit or mouse serum (Sigma–Aldrich) were injected i.p. or i.v. via the tail vein into groups of mice. Three days later, kidneys were harvested and stained for retained protein by immunofluorescence. Solutions of BSA (Sigma–Aldrich), human serum albumin (ZLB; Behring), and human IgG (Baxter) were diluted to the appropriate concentration in PBS and were 0.2-μm-filter sterilized. Before injection, solutions were centrifuged at 14,000 × g in a table-top centrifuge to precipitate large aggregates.
Immunofluorescence Studies.
Kidneys were embedded in Tissue-Tek OCT compound (Sakura Finetek) and snap-frozen in a dry-ice-cooled ethanol bath; 7-μm fresh-frozen sections were applied to SuperFrost slides (Fisher Scientific). Tissue patches were fixed in cold acetone and allowed to air dry. Kidney sections were encircled with a PAP pen to create a hydrophobic barrier and then blocked with 1% BSA in PBS. Rabbit, mouse, and human IgG were detected by using FITC- or Cy3-labeled antisera (Jackson ImmunoResearch). Images were captured on a Nikon Eclipse E800 system.
Nephrotoxic Serum Injections.
Four-month-old 129 × 1/J female mice were injected with either PBS or 50 mg of HSA i.p. on days 0 and 1. On days 4 and 5, mice were injected via the tail vein with 3.5 μl/g of either nephrotoxic or control serum diluted 2-fold in PBS to ensure accurate delivery of the required volume. The nephrotoxic serum has been described previously (26). Urine was collected on day 11 for dipstick analysis (Albustix; Bayer). Proteinuria greater than trace amounts (≥30 mg/dl) was scored as positive.
Isolation of Primary Podocytes and Microarray Analysis.
Mouse glomeruli were isolated from 3-month-old wild-type C57BL/6 mice by using Dynabead perfusion (41). Isolated glomeruli were incubated with trypsin solution containing 0.2% trypsin-EDTA (Sigma–Aldrich), 100 μg/ml heparin, and 100 units per ml DNase I in PBS for 25 min at 37°C. The trypsin was inactivated with soybean trypsin inhibitor (Sigma–Aldrich), and the cell suspension was sieved through a 30-μm-pore-size filter (BD Biosciences) as described previously (42). Cells were collected by centrifugation at 200 × g for 5 min at 4°C and resuspended in 500 μl of PBS supplemented with 0.1% BSA. The single-cell suspension was incubated with rabbit polyclonal antibody against the podocyte-specific membrane protein podocalyxin and goat anti-rabbit Alexafluor 488 secondary antibody (Invitrogen). Fluorophore-labeled podocytes were isolated by cell sorting. RNA was extracted from 6,000 positive cells obtained from eight mice with an RNeasy kit (Qiagen). A biotinylated cRNA target was generated and hybridized to an Affymetrix 430v2 murine genome array. Microarray data were analyzed by using dChip 2005 software (43). Datasets have been deposited in the Gene Expression Omnibus (GEO) database.
Generation and Culture of FcRn Wild-Type and Knockout Podocytes.
Conditionally immortalized podocytes from FcRn wild-type and knockout mice were generated as described previously (44). Podocytes were identified by their expression of the podocyte markers WT1, synaptopodin, and nephrin. Podocytes were cultured on collagen I-coated plates at 33°C in a growth-permissive medium of RPMI supplemented with 10% FBS and 100 units per ml of recombinant mouse IFNγ (a gift from Robert Schreiber, Washington University, St. Louis, MO). For differentiation, cells were shifted to 37°C, and the medium was changed to RPMI medium supplemented with 5% FBS without rIFNγ. Under these conditions, the cells' growth arrested and acquired a flatter morphology with extensive processes. Cells were incubated for a minimum of 10 days at 37°C before experiments or for genechip analysis.
Generation and Culture of Primary Mesangial Cells.
Primary mesangial cell cultures from FcRn wild-type and knockout mice were generated as described previously (45). Mesangial cells were grown from isolated glomeruli in RPMI medium 1640 supplemented with 20% FBS, 100 units per ml of penicillin/streptomycin, and ITS supplement (Sigma–Aldrich). The high-FBS concentration prevents the growth of podocytes. Glomeruli were cultured for 3 weeks until the mesangial cells became confluent. Mesangial cells possessed a stellate morphology when subconfluent and became more elongated when confluent. Cells were used at passages 2–4.
Generation of Anti-FcRn Antiserum.
A peptide spanning the α2–α3 region of mouse FcRn (amino acids 82–316) was PCR-amplified and cloned downstream of GST in pGEX4T-1 and expressed as a bacterial-inclusion body. The purified-inclusion body was solubilized in 6 M guanidine, and two rabbits were immunized with recombinant GST-FcRn (Sigma–Aldrich). Immune serum was affinity-purified on inclusion body protein blotted onto nitrocellulose.
Immunoblotting.
Liver tissue was homogenized in lysis buffer [1% TX-100, 20 mM Tris (pH 7.5), 50 mM NaCl, 50 mM NaF, 15 mM Na4P2O7, and 0.1 mM EDTA with protease inhibitors] by using a tissue homogenizer. Liver tissue or 5 × 106 podocytes from FcRn wild-type and knockout mice were lysed in lysis buffer for 20 min on ice. Nuclei were spun down at 500 × g for 5 min on a table-top centrifuge. The protein content of lysates was quantitated by using a BCA kit (Pierce). Equivalent amounts of protein from each sample were mixed with sample buffer and separated on a 10% SDS/PAGE gel. The proteins were transferred to nitrocellulose and immunoblotted for FcRn and α-tubulin as a loading control. Images were captured by using infrared imaging on a Licor Odyssey system.
I125 Labeling and Injections.
Human IgG (Baxter) was labeled with I125 by the Iodogen method; 2 × 106 cpm of labeled IgG (≈2 μg) in 200 μl of sterile PBS was injected i.v. into groups of 2- to 3-month-old sex-matched mice. One week later, mice were bled via the retroorbital sinus and were then killed by carbon dioxide inhalation followed by cervical dislocation. The kidneys were harvested, and the kidney capsule was removed. Each intact kidney was weighed and then placed into a separate scintillation tube for measurement of radioactivity. Undegraded IgG in serum was precipitated by mixing 50 μl of 20% TCA with 50 μl of serum. After mixing, the precipitate was spun down for 10 min at maximum speed in a table-top microcentrifuge, and the supernatant was discarded. The tip of the tube containing the pellet was cut off with a razor blade and placed into a scintillation tube for radioactivity measurements.
ACKNOWLEDGMENTS.
We thank George Jarad, Shirley Petzold, and Boris Calderon for technical assistance. This work was supported by National Institutes of Health Medical Science Training Program Grant T32 GM07200 and National Institute of Diabetes and Digestive and Kidney Grants 52701 and 56597.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tryggvason K, Wartiovaara J. How does the kidney filter plasma? Physiology (Bethesda) 2005;20:96–101. doi: 10.1152/physiol.00045.2004. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar MG, Wissig SL, Palade GE. Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J Exp Med. 1961;113:47–66. doi: 10.1084/jem.113.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karnovsky MJ, Ainsworth SK. The structural basis of glomerular filtration. Adv Nephrol Necker Hosp. 1972;2:35–60. [PubMed] [Google Scholar]
- 4.Miner JH. Renal basement membrane components. Kidney Int. 1999;56:2016–2024. doi: 10.1046/j.1523-1755.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- 5.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 6.Wartiovaara J, et al. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest. 2004;114:1475–1483. doi: 10.1172/JCI22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kestila M, et al. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 8.Smithies O. Why the kidney glomerulus does not clog: A gel permeation/diffusion hypothesis of renal function. Proc Natl Acad Sci USA. 2003;100:4108–4113. doi: 10.1073/pnas.0730776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adler S, Couser W. Immunologic mechanisms of renal disease. Am J Med Sci. 1985;289:55–60. doi: 10.1097/00000441-198502000-00003. [DOI] [PubMed] [Google Scholar]
- 10.McCluskey RT. Immunopathogenetic mechanisms in renal disease. Am J Kidney Dis. 1987;10:172–180. doi: 10.1016/s0272-6386(87)80171-3. [DOI] [PubMed] [Google Scholar]
- 11.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 12.Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985;15:733–738. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- 13.Rodewald R, Abrahamson DR. Receptor-mediated transport of IgG across the intestinal epithelium of the neonatal rat. Ciba Found Symp. 1982;92:209–232. doi: 10.1002/9780470720745.ch11. [DOI] [PubMed] [Google Scholar]
- 14.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. Increased clearance of IgG in mice that lack beta 2-microglobulin: Possible protective role of FcRn. Immunology. 1996;89:573–578. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghetie V, et al. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 17.Roopenian DC, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgE-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhury C, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward ES, Zhou J, Ghetie V, Ober RJ. Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int Immunol. 2003;15:187–195. doi: 10.1093/intimm/dxg018. [DOI] [PubMed] [Google Scholar]
- 20.Haymann JP, et al. Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol. 2000;11:632–639. doi: 10.1681/ASN.V114632. [DOI] [PubMed] [Google Scholar]
- 21.Praetor A, Ellinger I, Hunziker W. Intracellular traffic of the MHC class I-like IgG Fc receptor, FcRn, expressed in epithelial MDCK cells. J Cell Sci. 1999;112(Pt 14):2291–2299. doi: 10.1242/jcs.112.14.2291. [DOI] [PubMed] [Google Scholar]
- 22.Dickinson BL, et al. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol. 2007;179:4580–4588. doi: 10.4049/jimmunol.179.7.4580. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi N, et al. FcRn-mediated transcytosis of immunoglobulin G in human renal proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2002;282:F358–F365. doi: 10.1152/ajprenal.0164.2001. [DOI] [PubMed] [Google Scholar]
- 25.Borvak J, et al. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol. 1998;10:1289–1298. doi: 10.1093/intimm/10.9.1289. [DOI] [PubMed] [Google Scholar]
- 26.Yo Y, et al. Anti-mouse mesangial cell serum induces acute glomerulonephropathy in mice. Nephron Exp Nephrol. 2003;93:e92–e106. doi: 10.1159/000069551. [DOI] [PubMed] [Google Scholar]
- 27.Haraldsson B, Sorensson J. Why do we not all have proteinuria? An update of our current understanding of the glomerular barrier. News Physiol Sci. 2004;19:7–10. doi: 10.1152/nips.01461.2003. [DOI] [PubMed] [Google Scholar]
- 28.Ohlson M, Sorensson J, Haraldsson B. A fel-membrane model of glomerular charge and size selectivity in series. Am J Physiol Renal Physiol. 2001;280:F396–F405. doi: 10.1152/ajprenal.2001.280.3.F396. [DOI] [PubMed] [Google Scholar]
- 29.Morigi M, et al. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: Implication for permselective dysfunction of chronic nephropathies. Am J Pathol. 2005;166:1309–1320. doi: 10.1016/S0002-9440(10)62350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh AK, Rahman MA. Intracellular processing of immune complexes formed on the surface of glomerular epithelial cells. Am J Physiol. 1994;266:F246–F253. doi: 10.1152/ajprenal.1994.266.2.F246. [DOI] [PubMed] [Google Scholar]
- 31.Eppel GA. The return of glomerular-filtered albumin to the rat renal vein. Kidney Int. 1999;55:1861–1870. doi: 10.1046/j.1523-1755.1999.00424.x. [DOI] [PubMed] [Google Scholar]
- 32.Eppel GA, et al. The return of glomerular filtered albumin to the rat renal vein–the albumin retrieval pathway. Ren Fail. 2001;23:347–363. doi: 10.1081/jdi-100104719. [DOI] [PubMed] [Google Scholar]
- 33.Russo LM, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int. 2007;71:504–513. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 34.Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol. 1992;263:F601–F606. doi: 10.1152/ajprenal.1992.263.4.F601. [DOI] [PubMed] [Google Scholar]
- 35.Ohlson M, et al. Effects of filtration rate on the glomerular barrier and clearance of four differently shaped molecules. Am J Physiol Renal Physiol. 2001;281:F103–F113. doi: 10.1152/ajprenal.2001.281.1.F103. [DOI] [PubMed] [Google Scholar]
- 36.Farquhar MG, Palade GE. Segregation of ferritin in glomerular protein absorption droplets. J Biophys Biochem Cytol. 1960;7:297–304. doi: 10.1083/jcb.7.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farquhar MG, Palade GE. Glomerular permeability II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med. 1961;114:699–716. doi: 10.1084/jem.114.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharon Z, Schwartz MM, Pauli BU, Lewis EJ. Kinetics of glomerular visceral epithelial cell phagocytosis. Kidney Int. 1978;14:526–529. doi: 10.1038/ki.1978.158. [DOI] [PubMed] [Google Scholar]
- 39.Lewis EJ, Schwartz MM, Pauli BU, Sharon Z. Endocytosis: A property of the glomerular visceral epithelial cell. Nephron. 1978;22:91–96. doi: 10.1159/000181427. [DOI] [PubMed] [Google Scholar]
- 40.Rantala I. Glomerular epithelial cell endocytosis of immune deposits in the nephrotic rat. An ultrastructural immunoperoxidase study. Nephron. 1981;29:239–244. doi: 10.1159/000182381. [DOI] [PubMed] [Google Scholar]
- 41.Takemoto M, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takemoto M, et al. Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J. 2006;25:1160–1174. doi: 10.1038/sj.emboj.7601014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mundel P, et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 45.Cattell V, et al. Anti-GBM glomerulonephritis in mice lacking nitric oxide synthase type 2. Kidney Int. 1998;53:932–936. doi: 10.1111/j.1523-1755.1998.00892.x. [DOI] [PubMed] [Google Scholar]