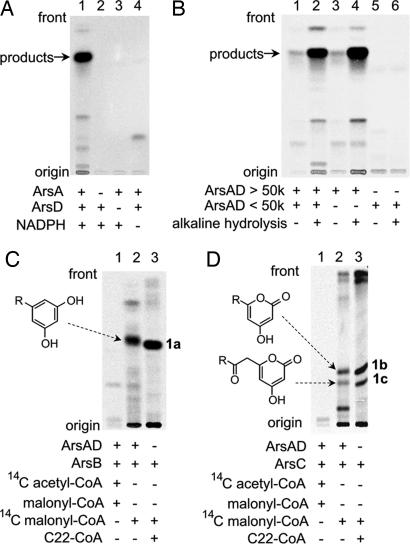
Fig. 4.
Radio-TLC analysis of in vitro reaction products. (A) Analysis of products produced from malonyl-CoA by ArsA and ArsD. The production of radiolabeled products was observed in the presence of [2-14C]malonyl-CoA, NADPH, ArsA, and ArsD (lane 1). No radioactivity was seen in the absence of ArsA (lane 2), ArsD (lane 3), or NADPH (lane 4). (B) Analysis of a hydrolyzed reaction mixture containing ArsA and ArsD. After incubation of malonyl-CoA in the presence of ArsA and ArsD, the reaction mixture was hydrolyzed by alkali, extracted with ethyl acetate, and subjected to TLC. Lane 1 is a negative control containing a complete reaction mixture that was not subjected to hydrolysis. [14C] attached to ArsA was fractionated into the water layer and did not appear in the TLC analysis. Lane 2 contains a complete reaction mixture that was hydrolyzed and extracted with ethyl acetate. Lanes 3 and 4 contain a macromolecular fraction of the reaction mixture that was hydrolyzed (lane 4) or not hydrolyzed (lane 3). Lanes 5 and 6 contain a low-molecular-weight fraction that was hydrolyzed (lane 6) or not hydrolyzed (lane 5). (C) Analysis of the products of ArsA, ArsB, and ArsD from two different starter substrates. The starter substrates used were [2-14C]acetyl-CoA (lane 1) and [2-14C]malonyl-CoA (lane 2). Lane 3 contains an authentic sample 1a prepared from behenyl-CoA (C22-CoA) and malonyl-CoA by ArsB. (D) Analysis of the products of ArsA, ArsC, and ArsD from two different starter substrates. The starter substrates used were [2-14C]acetyl-CoA (lane 1) and [2-14C]malonyl-CoA (lane 2). Lane 3 contains authentic samples 1b and 1c prepared from behenyl-CoA (C22-CoA) and malonyl-CoA by ArsC.