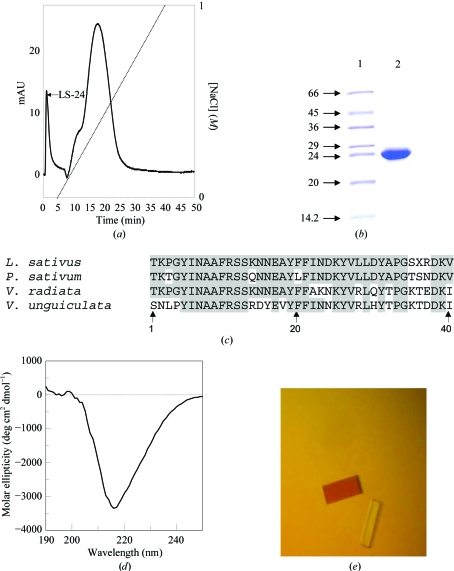
Figure 2.
Purification, characterization and crystallization of 24 kDa protein from seeds of L. sativus. (a) Purification of 24 kDa protein by ion-exchange chromatography on a PI/M column. The peak LS-24 corresponds to the 24 kDa protein from L. sativus. The dotted line indicates the NaCl gradient. (b) Purified 24 kDa protein on SDS–PAGE. Lanes 1 and 2 correspond to protein markers (kDa) and purified protein, respectively. (c) Sequence alignments of the 24 kDa protein of L. sativus with other legume albumins. Identical amino-acid residues are highlighted in dark grey. (d) CD spectra of the 24 kDa protein in the far-UV region. (e) Crystals of the 24 kDa protein grown by the hanging-drop method.