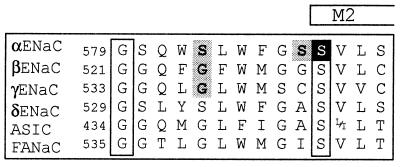
Figure 1.
Sequence alignment of the pre-M2 segments of α, β, γ, δ ENaC, the ASIC family, and FANaC [FMRFamide peptide-gated Na+ channel (8)] (2, 5). The number of the first residue shown of rat α, β, γ ENaC, human δ ENaC, rat ASIC1, and FANaC is indicated. The putative start of M2, as predicted by the phdhtm (transmembrane helix location) program at European Molecular Biology Laboratory (EMBL)–Heidelberg is indicated. The amino acid residues shown are identical within subunits across the species. Amino acid residues shown in boxes are conserved across all known genes of the ENaC family. ENaC αS589 is shown in white on a dark background. αS583, and the homologous amino acid residues βG525 and γG537, shown on gray background, change channel affinity for amiloride block (7). Mutation of αS588 (shown on gray background) to Ile has been shown to affect Na+/Li+ selectivity and amiloride binding (9).