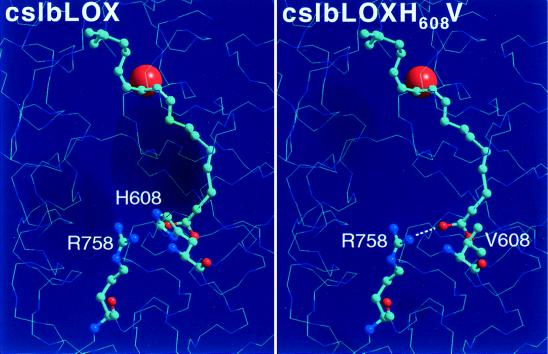
Figure 3.
Model showing inverse-substrate binding with respect to the proposed mode of binding of the lipid body LOX and its H608V mutant. (Left) Active-site model of the lipid body LOX. Here, the methyl terminus of LA is nearby the side chain of H608. The charged R758 is shielded by H608. (Right) Model for the H608V mutant. When oriented inversely, the negatively charged carboxylic group of LA (red) may form a salt bridge (dotted line) with positively charged nitrogens (blue) of R758.