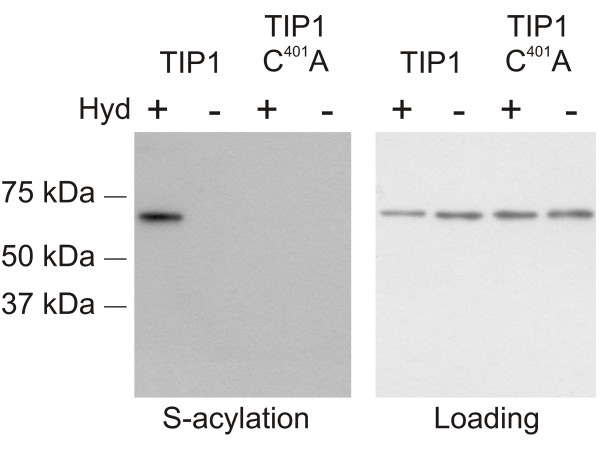
Figure 2.
The biotin switch protocol detects C401A-dependent S-acylation of TIP1. ysates of yeast expressing TIP1, or the point mutant TIP1 C401A, were put through the biotin switch protocol summarised in Figure 1. Samples were treated with (Hyd+) or without (Hyd-) the thioester cleavage reagent hydroxylamine. The loading controls show that equal amounts of TIP1 and TIP1 C401A were loaded onto neutravidin beads. The lanes labelled 'S-acylation' show the levels of TIP1 and TIP1 C401A recovered from the neutravidin beads, and therefore originally S-acylated. TIP1 and TIP1 C401A have masses of 70 kDa. The results show that Arabidopsis TIP1 binds acyl groups by a covalent thioester bond but TIP1 C401A does not, consistent with previous results using a H3-palmitic acid S-acylation assay [18].