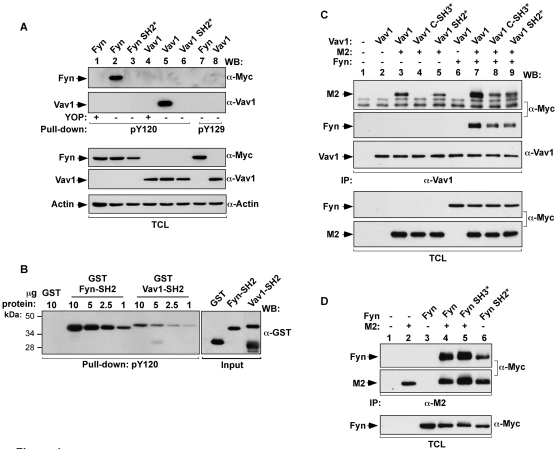
Figure 4. Phosphorylated M2 binds directly to SH2 domains of Fyn and Vav1.
(A) COS1 cells were transiently transfected with plasmids encoding the indicated proteins (top). Cell extracts were incubated with biotinylated peptides containing M2 sequences encompassing phosphorylated (−) and YOP-mediated (+) dephosphorylated versions of residues 120 and 129. Complexes were recovered with streptavidin-coupled agarose beads and analyzed by western blot using the indicated antibodies (right side of panels) to reveal the possible association of the indicated versions of Fyn (top panel) and Vav1 (second panel from top). Western blot analysis of aliquots of TCL confirmed expression of proteins (three bottom panels). (B) The phosphorylated Y120 peptide described in (A) was incubated with increasing amounts of the indicated GST fusions proteins purified from bacteria. After binding, the peptide was recovered as indicated above and bound proteins identified by immunoblot analysis using anti-GST antibodies (upper panel on the left). Aliquots of GST proteins were analyzed by western blotting (upper panel on the right). (C,D) COS1 cells were transiently transfected with plasmids encoding the indicated proteins. Clarified lysates were incubated with anti-Vav1 (C) or anti-M2 (D) antibodies. Immunoprecipitates were analyzed by western blotting using the indicated antibodies (left). Aliquots of TCL used in the immunoprecipitations were analysed in parallel to confirm the expression of proteins used in this experiment (bottom panels). *, point mutation in the indicated domain of Fyn or Vav1; −, without; +, with; IP, immunoprecipitation; WB, western blotting.