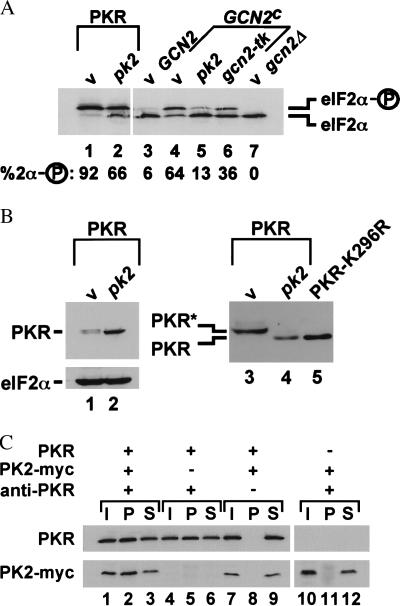
Figure 3.
Inhibition of eIF2α phosphorylation in yeast cells by expression of pk2 and coprecipitation of PK2 with PKR. (A) Analysis of eIF2α phosphorylation. Plasmids encoding pk2 or gcn2-tk or an empty vector (v) were introduced into yeast strains expressing PKR, wild-type GCN2, hyperactive GCN2c-E532K-E1522K (GCN2c), or no eIF2α kinase (gcn2), as indicated. Crude protein extracts were prepared and analyzed by IEF gel electrophoresis on a polyacrylamide slab gel followed by immunoblotting with yeast eIF2α-specific antiserum. The eIF2α phosphorylated on Ser-51 focuses above the protein lacking the Ser-51 phosphorylation, as indicated. The percentage of eIF2α that is phosphorylated on Ser-51 was determined by quantitative densitometry and is indicated below each lane. (B) Analysis of PKR expression. (Lanes 1 and 2) PKR expression in the strains shown in A, lanes 1 and 2, was analyzed by immunoblotting 50 μg of whole cell extracts and probing with antiserum specific for yeast eIF2α or with human PKR monoclonal antibodies, as indicated. (Lanes 3–5) Crude protein extracts from an eIF2α-S51A yeast strain expressing PKR, PK2, or PKR-K296R, as indicated, were subjected to SDS/PAGE on 7.5% gels and then immunoblotted with human PKR monoclonal antibodies. The phosphorylated form of PKR (PKR*) migrates slower than the unphosphorylated form (PKR), as indicated. The slower mobility of wild-type PKR, compared with inactive PKR-K296R, in SDS/PAGE is due to extensive autophosphorylation of the wild-type protein. Treatment of wild-type PKR with phosphatase increases its mobility and causes wild-type PKR to comigrate with nonphosphorylated PKR-K296R (ref. 35; P. Romano and A. Hinnebusch, personal communication). (C) Coprecipitation of PKR and PK2. Crude protein extracts from yeast strains expressing PKR-K296R (PKR) and c-myc epitope-tagged pk2 (PK2-myc), as indicated, were incubated with anti-PKR polyclonal antiserum bound to protein A-agarose beads; after precipitation, fractions of the starting materials (I), pellets (P), and supernatants (S) were subjected to SDS/PAGE followed by immunoblotting with human PKR or c-myc monoclonal antibodies, as indicated.