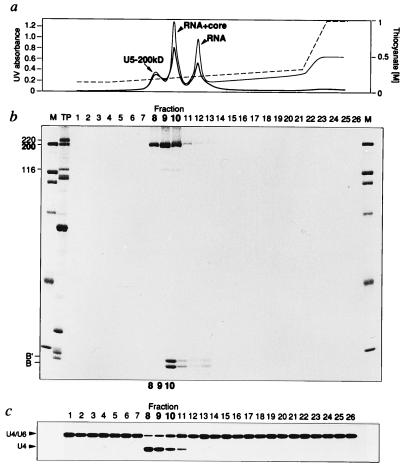
Figure 4.
Highly purified U5–200kD protein successively fractionated on NaSCN-glycerol gradients and by MonoQ chromatography is still active in U4/U6 RNA duplex unwinding. (a) Elution profile from the MonoQ column. The absorbance of the eluted fractions is measured at 280 nm (thick line) and 260 nm (thin line). The content of the peak eluates is indicated. The NaSCN concentrations are shown to the right, and the step gradient for elution is displayed by a broken line. (b) Coomassie-staining of the proteins present in the eluted fractions. M, molecular mass standards of 200, 116, 97, 66, 45, and 34 kDa. TP, total proteins from a mixture of spliceosomal snRNPs. (c) RNA unwinding activity of the eluted fractions. After dialysis against G-buffer with 5% glycerol, equivalent volumes of the eluted fractions were assayed for U4/U6 RNA unwinding in the presence of 200 μM ATP.