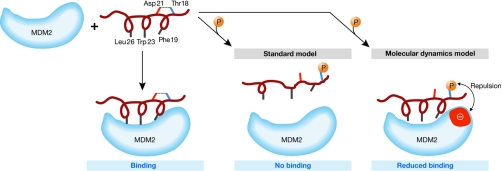
Figure 3.
Schematic of the binding of p53 amino-terminal helical peptide to MDM2. The p53 hydrophobic residues, Phe 19, Trp 23 and Leu 26, interact with the MDM2 binding pocket. The standard model suggests that the hydrogen bond (dotted line) between Thr 18 and Asp 21 is responsible for the maintenance of the helical motif necessary to maximize the interactions with MDM2. On phosphorylation of Thr 18, the hydrogen bond with Asp 21 is broken and the helix is destabilized, resulting in disruption of the p53–MDM2 interaction. The simulations of molecular dynamics by Lee et al (2007) suggest that, although the hydrogen bond is broken on phosphorylation, the helix is marginally destabilized and the loss of binding also arises from repulsions generated between the negatively charged phosphate group and an anionic patch (red) on the surface of MDM2 in the vicinity of the phosphorylated Thr 18. MDM2, ubiquitin E3 ligase.