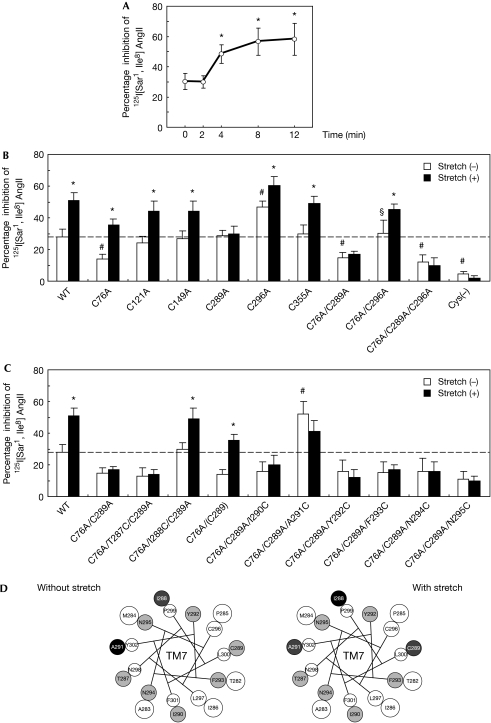
Figure 1.
Mechanical stress-induced anticlockwise rotation of TM7 in the AT1 receptor. (A) Alteration of cysteine accessibility by mechanical stretch in HEK293-AT1 cells. Cell membranes were prepared before (0 min) and after the indicated stretch time, and subjected to a SCAM study. *P<0.05 versus 0 min. (B) Alteration of cysteine accessibility by mechanical stretch in COS7 cells expressing wild-type (WT) and mutant AT1 receptors. The cells were stretched for 8 min. Cys(−) represents a mutant receptor in which all the cysteine residues were replaced with alanine. *P<0.05 versus stretch (−), #P<0.05 versus stretch (−) in wild-type, §P<0.05 versus stretch (−) in Cys76Ala/Cys289Ala. (C) Alteration of cysteine accessibility by mechanical stretch in COS7 cells expressing Cys76Ala/Cys289Ala mutant receptors in which TM7 residues ranging from Thr 287 to Asn 295 were successively substituted to cysteine. *P<0.05 versus stretch (−), #P<0.05 versus stretch (−) in Cys76Ala/Cys289Ala. (D) Helical wheel representation of TM7 reporter cysteine residues and the pattern of their reactivity to MTSEA+. Positions of MTSEA+-reacted cysteine residues in TM7 that affected 125I-labelled (Sar1, Ile8) AngII binding are shown in a helical wheel representation viewed from the extracellular side without (left) or with (right) stretch. Black circles correspond to the residues that inhibited 125I-labelled (Sar1, Ile8) AngII binding by 50% or more when substituted to cysteine, whereas dark grey circles indicate those that inhibited by around 30%. Light grey circles indicate those that had no inhibitory effect. White circles indicate receptors that were not examined. AngII, angiotensin II; AT1, AngII type 1; MTSEA+, methanethiosulphonate ethyl-ammonium; SCAM, substituted cysteine accessibility mapping; TM7, transmembrane 7.