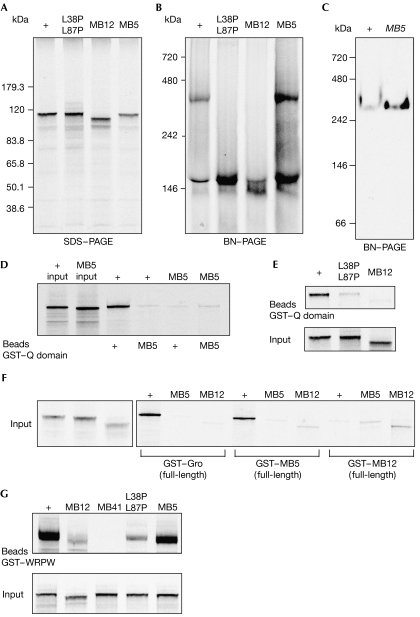
Figure 5.
Q-domain-mediated oligomerization of Gro variants. (A) Autoradiograph of 35S-Met-labelled Gro mutant proteins run on SDS–polyacrylamide gel electrophoresis. MB12 migrates slightly faster than wild type (+), which is consistent with an amino-terminal truncation. MB5 and L36P,L87P variants run similar to wild type. (B) Autoradiograph of the translation reactions shown in (A) run on BN-PAGE. MB12 and L36P,L87P do not form a tetrameric complex. (C) Western blot of wild type and MB5 extracts run on BN-PAGE. MB5 forms a high-molecular-weight, tetrameric band. (D,E) Pull-down analysis of 35S-Met-labelled full-length, translated Gro+ and (D) MB5 or (E) L36P,L87P or MB12 protein on GST–wild-type and GST–MB5 Q domains. Neither MB5 nor MB12 binds to an isolated Q domain in this assay. (F) Pull-down analysis of translated Gro+, MB5 and MB12 proteins on GST fusions of full-length Gro+, MB5 and MB12 proteins, showing that immobilized, full-length MB5 retains the ability to bind to translated Gro+. (G) Disruption of oligomerization in two different Q domain mutants, MB12 and L38P,L87P, reduces binding to the WRPW motif. MB41 is a missense mutation in the WD domain that abolishes binding to WRPW (Jennings et al, 2006). Control input tracks represent 10% of labelled Gro protein. BN-PAGE, blue native-polyacrylamide gel electrophoresis; Gro, Groucho; GST, glutathione-S-transferase.