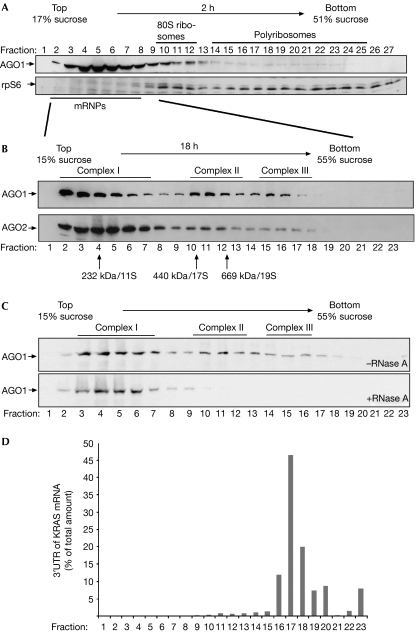
Figure 1.
Human AGO1 and AGO2 associate with distinct protein–RNA complexes. (A) Individual fractions of polyribosome gradients were analysed by western blotting against endogenous AGO1 (upper panel) or rpS6 (lower panel). (B) Lysates from wild-type HEK 293 cells were separated by sucrose density centrifugation under conditions that allow the separation of mRNPs. Endogenous AGO1 and AGO2 were analysed using specific antibodies. (C) Lysates were analysed as in (B). Lysates shown in the lower panel were treated with 100 μg/ml RNase A before centrifugation. (D) A reporter construct containing the KRAS 3′-UTR was transfected into HEK 293 cells and lysates were separated as in (B). Total RNA was extracted from the individual fractions and analysed by qRT–PCR. The distribution of the KRAS 3′-UTR is shown as a percentage of the total amount of the KRAS 3′-UTR. AGO, Argonaute; HEK, human embryonic kidney; KRAS, Kirsten rat sarcoma viral oncogene homologue; mRNPs, messenger ribonucleoproteins; qRT–PCR, quantitative reverse transcription–PCR; rpS6, ribosomal protein S6; 3′-UTR, 3′-untranslated region.